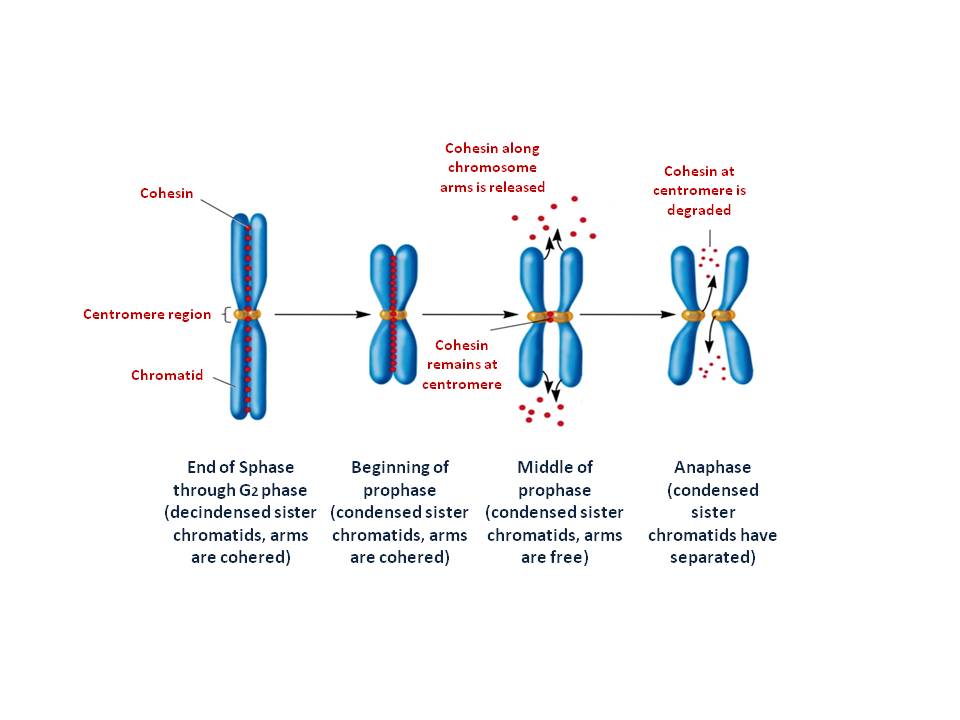
[1]
Travers A, Muskhelishvili G. DNA structure and function. The FEBS journal. 2015 Jun:282(12):2279-95. doi: 10.1111/febs.13307. Epub 2015 Jun 2
[PubMed PMID: 25903461]
[2]
Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997 Sep 18:389(6648):251-60
[PubMed PMID: 9305837]
[3]
Mariño-Ramírez L, Kann MG, Shoemaker BA, Landsman D. Histone structure and nucleosome stability. Expert review of proteomics. 2005 Oct:2(5):719-29
[PubMed PMID: 16209651]
[4]
Kornberg RD. Chromatin structure: a repeating unit of histones and DNA. Science (New York, N.Y.). 1974 May 24:184(4139):868-71
[PubMed PMID: 4825889]
[6]
Tessarz P, Kouzarides T. Histone core modifications regulating nucleosome structure and dynamics. Nature reviews. Molecular cell biology. 2014 Nov:15(11):703-8. doi: 10.1038/nrm3890. Epub 2014 Oct 15
[PubMed PMID: 25315270]
[7]
Gallinari P, Di Marco S, Jones P, Pallaoro M, Steinkühler C. HDACs, histone deacetylation and gene transcription: from molecular biology to cancer therapeutics. Cell research. 2007 Mar:17(3):195-211
[PubMed PMID: 17325692]
[8]
Katan-Khaykovich Y, Struhl K. Heterochromatin formation involves changes in histone modifications over multiple cell generations. The EMBO journal. 2005 Jun 15:24(12):2138-49
[PubMed PMID: 15920479]
[9]
Pinel C, Prainsack B, McKevitt C. Markers as mediators: A review and synthesis of epigenetics literature. BioSocieties. 2019 May 10:13(1):276-303. doi: 10.1057/s41292-017-0068-x. Epub 2017 Sep 18
[PubMed PMID: 31105763]
[10]
Fangman WL, Brewer BJ. A question of time: replication origins of eukaryotic chromosomes. Cell. 1992 Oct 30:71(3):363-6
[PubMed PMID: 1423601]
[11]
Kulis M, Esteller M. DNA methylation and cancer. Advances in genetics. 2010:70():27-56. doi: 10.1016/B978-0-12-380866-0.60002-2. Epub
[PubMed PMID: 20920744]
Level 3 (low-level) evidence
[12]
McGinty RK, Tan S. Nucleosome structure and function. Chemical reviews. 2015 Mar 25:115(6):2255-73. doi: 10.1021/cr500373h. Epub 2014 Dec 12
[PubMed PMID: 25495456]
[13]
Kalashnikova AA, Rogge RA, Hansen JC. Linker histone H1 and protein-protein interactions. Biochimica et biophysica acta. 2016 Mar:1859(3):455-61. doi: 10.1016/j.bbagrm.2015.10.004. Epub 2015 Oct 8
[PubMed PMID: 26455956]
[14]
Smith BC, Denu JM. Chemical mechanisms of histone lysine and arginine modifications. Biochimica et biophysica acta. 2009 Jan:1789(1):45-57. doi: 10.1016/j.bbagrm.2008.06.005. Epub 2008 Jun 14
[PubMed PMID: 18603028]
[15]
Dueva R, Akopyan K, Pederiva C, Trevisan D, Dhanjal S, Lindqvist A, Farnebo M. Neutralization of the Positive Charges on Histone Tails by RNA Promotes an Open Chromatin Structure. Cell chemical biology. 2019 Oct 17:26(10):1436-1449.e5. doi: 10.1016/j.chembiol.2019.08.002. Epub 2019 Aug 22
[PubMed PMID: 31447351]
[16]
Davey CA, Sargent DF, Luger K, Maeder AW, Richmond TJ. Solvent mediated interactions in the structure of the nucleosome core particle at 1.9 a resolution. Journal of molecular biology. 2002 Jun 21:319(5):1097-113
[PubMed PMID: 12079350]
[17]
Rohs R, West SM, Sosinsky A, Liu P, Mann RS, Honig B. The role of DNA shape in protein-DNA recognition. Nature. 2009 Oct 29:461(7268):1248-53. doi: 10.1038/nature08473. Epub
[PubMed PMID: 19865164]
[18]
Tremethick DJ. Higher-order structures of chromatin: the elusive 30 nm fiber. Cell. 2007 Feb 23:128(4):651-4
[PubMed PMID: 17320503]
[20]
Chen P, Li G. Dynamics of the higher-order structure of chromatin. Protein & cell. 2010 Nov:1(11):967-71. doi: 10.1007/s13238-010-0130-y. Epub
[PubMed PMID: 21153512]
[21]
Kruithof M, Chien FT, Routh A, Logie C, Rhodes D, van Noort J. Single-molecule force spectroscopy reveals a highly compliant helical folding for the 30-nm chromatin fiber. Nature structural & molecular biology. 2009 May:16(5):534-40. doi: 10.1038/nsmb.1590. Epub 2009 Apr 19
[PubMed PMID: 19377481]
[22]
Luger K, Dechassa ML, Tremethick DJ. New insights into nucleosome and chromatin structure: an ordered state or a disordered affair? Nature reviews. Molecular cell biology. 2012 Jun 22:13(7):436-47. doi: 10.1038/nrm3382. Epub 2012 Jun 22
[PubMed PMID: 22722606]
[23]
Kireeva N, Lakonishok M, Kireev I, Hirano T, Belmont AS. Visualization of early chromosome condensation: a hierarchical folding, axial glue model of chromosome structure. The Journal of cell biology. 2004 Sep 13:166(6):775-85
[PubMed PMID: 15353545]
[24]
Cremer T, Cremer M. Chromosome territories. Cold Spring Harbor perspectives in biology. 2010 Mar:2(3):a003889. doi: 10.1101/cshperspect.a003889. Epub
[PubMed PMID: 20300217]
Level 3 (low-level) evidence
[25]
Cockerill PN, Garrard WT. Chromosomal loop anchorage of the kappa immunoglobulin gene occurs next to the enhancer in a region containing topoisomerase II sites. Cell. 1986 Jan 31:44(2):273-82
[PubMed PMID: 3002631]
[26]
Heng HH, Goetze S, Ye CJ, Liu G, Stevens JB, Bremer SW, Wykes SM, Bode J, Krawetz SA. Chromatin loops are selectively anchored using scaffold/matrix-attachment regions. Journal of cell science. 2004 Mar 1:117(Pt 7):999-1008
[PubMed PMID: 14996931]
[27]
Narwade N, Patel S, Alam A, Chattopadhyay S, Mittal S, Kulkarni A. Mapping of scaffold/matrix attachment regions in human genome: a data mining exercise. Nucleic acids research. 2019 Aug 22:47(14):7247-7261. doi: 10.1093/nar/gkz562. Epub
[PubMed PMID: 31265077]
[28]
Bouazzi H, Thakur S, Trujillo C, Alwasiyah MK, Munnich A. Novel ATRX gene damaging missense mutation c.6740A}C segregates with profound to severe intellectual deficiency without alpha thalassaemia. The Indian journal of medical research. 2016 Jan:143(1):43-8. doi: 10.4103/0971-5916.178589. Epub
[PubMed PMID: 26997013]
[29]
Iwase S, Bérubé NG, Zhou Z, Kasri NN, Battaglioli E, Scandaglia M, Barco A. Epigenetic Etiology of Intellectual Disability. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2017 Nov 8:37(45):10773-10782. doi: 10.1523/JNEUROSCI.1840-17.2017. Epub
[PubMed PMID: 29118205]
[30]
Berdasco M, Esteller M. Genetic syndromes caused by mutations in epigenetic genes. Human genetics. 2013 Apr:132(4):359-83. doi: 10.1007/s00439-013-1271-x. Epub 2013 Jan 31
[PubMed PMID: 23370504]
[31]
Wada T. [X-linked alpha-thalassemia/mental retardation syndrome]. Rinsho byori. The Japanese journal of clinical pathology. 2009 Apr:57(4):382-90
[PubMed PMID: 19489441]
[32]
Olson CO, Pejhan S, Kroft D, Sheikholeslami K, Fuss D, Buist M, Ali Sher A, Del Bigio MR, Sztainberg Y, Siu VM, Ang LC, Sabourin-Felix M, Moss T, Rastegar M. MECP2 Mutation Interrupts Nucleolin-mTOR-P70S6K Signaling in Rett Syndrome Patients. Frontiers in genetics. 2018:9():635. doi: 10.3389/fgene.2018.00635. Epub 2018 Dec 19
[PubMed PMID: 30619462]
[33]
Urdinguio RG, Sanchez-Mut JV, Esteller M. Epigenetic mechanisms in neurological diseases: genes, syndromes, and therapies. The Lancet. Neurology. 2009 Nov:8(11):1056-72. doi: 10.1016/S1474-4422(09)70262-5. Epub
[PubMed PMID: 19833297]
[34]
Rozek LS, Dolinoy DC, Sartor MA, Omenn GS. Epigenetics: relevance and implications for public health. Annual review of public health. 2014:35():105-22. doi: 10.1146/annurev-publhealth-032013-182513. Epub
[PubMed PMID: 24641556]
[35]
Sen N. Epigenetic regulation of memory by acetylation and methylation of chromatin: implications in neurological disorders, aging, and addiction. Neuromolecular medicine. 2015 Jun:17(2):97-110. doi: 10.1007/s12017-014-8306-x. Epub 2014 Apr 29
[PubMed PMID: 24777294]
[36]
Hudson AJ. Consciousness and cell memory: a dynamic epigenetic interrelationship. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 2011 Sep:38(5):681-8
[PubMed PMID: 21856569]
[37]
Perri F, Longo F, Giuliano M, Sabbatino F, Favia G, Ionna F, Addeo R, Della Vittoria Scarpati G, Di Lorenzo G, Pisconti S. Epigenetic control of gene expression: Potential implications for cancer treatment. Critical reviews in oncology/hematology. 2017 Mar:111():166-172. doi: 10.1016/j.critrevonc.2017.01.020. Epub 2017 Feb 4
[PubMed PMID: 28259291]
[38]
Derissen EJ, Beijnen JH, Schellens JH. Concise drug review: azacitidine and decitabine. The oncologist. 2013:18(5):619-24. doi: 10.1634/theoncologist.2012-0465. Epub 2013 May 13
[PubMed PMID: 23671007]
[39]
Haas NB,Quirt I,Hotte S,McWhirter E,Polintan R,Litwin S,Adams PD,McBryan T,Wang L,Martin LP,vonMehren M,Alpaugh RK,Zweibel J,Oza A, Phase II trial of vorinostat in advanced melanoma. Investigational new drugs. 2014 Jun;
[PubMed PMID: 24464266]
[40]
Cheng Y, He C, Wang M, Ma X, Mo F, Yang S, Han J, Wei X. Targeting epigenetic regulators for cancer therapy: mechanisms and advances in clinical trials. Signal transduction and targeted therapy. 2019:4():62. doi: 10.1038/s41392-019-0095-0. Epub 2019 Dec 17
[PubMed PMID: 31871779]
Level 3 (low-level) evidence