Continuing Education Activity
Measles, also known as rubeola, is a preventable, highly contagious, acute febrile viral illness. It remains an important cause of global mortality and morbidity, particularly in the regions of Africa and Southeast Asia. It accounts for about 100,000 deaths annually despite the availability of an effective vaccine. Public health officials declared the elimination of measles from the U.S. in 2000, marking the absence of continuous disease transmission for one year and from the region of the Americas in 2016. However, outbreaks continue to occur through imported disease and transmission among unvaccinated groups of children in the community. According to the Center for Disease Control (CDC), there were 372 cases in 2018 and 764 cases through May of 2019. This activity provides an overview of measles prevention, transmission, and diagnosis and highlights the role of the interprofessional team in helping their patients prevent this disease.
Objectives:
Describe the pathophysiology of measles.
Describe the epidemiology of measles.
Summarize the role of vaccination in the prevention of measles.
Describe the importance of communication between the interprofessional care teams and patients to improve vaccination rates and thereby limit morbidity and mortality associated with measles.
Introduction
Measles, also known as rubeola, is a preventable, highly contagious, acute febrile viral illness. It remains an important cause of global mortality and morbidity, particularly in the regions of Africa and Southeast Asia.[1][2] It accounts for about 100,000 deaths annually despite the availability of an effective vaccine. Public health officials declared the elimination of measles from the U.S. in 2000, marking the absence of continuous disease transmission for one year and from the region of the Americas in 2016. However, outbreaks continue to occur through imported disease and transmission among unvaccinated groups of children in the community. Measles is a reportable disease in most nations, including the United States.[3]
Etiology
The causative organism is the measles virus, a member of the Paramyxoviridae family and Morbillivirus genus. It is an enveloped, single-stranded, nonsegmented, negative-sense RNA virus. The genome encodes six structural proteins and two non-structural proteins, V and C. The structural proteins are nucleoprotein, phosphoprotein, matrix, fusion, haemagglutinin (HA), and large protein. The HA protein is responsible for virus attachment to the host cell.[4]
Epidemiology
The epidemiology of measles is variable across the globe and is related to immunization levels achieved in a particular region. Before implementing widespread vaccination programs, measles accounted for an estimated 2.6 million deaths. Despite vaccination in the present era, The World Health Organization (WHO) reported that approximately 134,200 deaths (15 deaths/hour) occurred in 2015 due to measles. According to the CDC, there were 372 cases in 2018 and 764 cases through May of 2019. Measles is a reportable disease in most nations, including the United States.
The measles virus has no animal reservoir and occurs only in humans. The virus is highly contagious, with each case capable of causing 14 to 18 secondary cases among susceptible populations. Measles is transmitted from person to person by respiratory droplets, small particle aerosols, and close contact. The incubation period is 10 to 14 days, although longer periods have been reported. Unvaccinated young children and pregnant women are at high risk for contracting measles, and measles most commonly affects young children. More recently, there has been a shift to older children and adolescents due to increasing levels of immunization coverage and alterations in the levels of population immunity at different ages. Young infants born to mothers with acquired immunity are protected from measles due to passive antibody transfer, but as these antibodies wane, they become susceptible. Infectiousness of a case is maximal in the four days before and four days after the rash develops, which coincides with peak levels of viremia and the features of cough, conjunctivitis, and coryza.[5][6][7][8]
Pathophysiology
The inhaled virus from the exposed droplets initially infects the respiratory tract’s lymphocytes, dendritic cells, and alveolar macrophages. It then spreads to the adjacent lymphoid tissue and disseminates throughout the bloodstream resulting in viremia and spreading to distant organs. The virus residing in the dendritic cells and lymphocytes transfers itself to the epithelial cells of the respiratory tract, which are shed and expelled as respiratory droplets during coughing and sneezing, infecting others and perpetuating the cycle. The initial inflammation leads to symptoms of coryza, conjunctivitis, and cough. The appearance of fever coincides with the development of viremia. The skin rash occurs after dissemination and is due to perivascular and lymphocytic infiltrates.
During the prodromal phase, the measles virus depresses host immunity by suppressing interferon production through its nonstructural proteins, V and C. The increasing viral replication then triggers both humoral and cellular immunological responses. The initial humoral response consists of IgM antibody production, which is detectable 3 to 4 days after the rash appears and can persist for 6 to 8 weeks. Subsequently, IgG antibodies are produced, primarily against the viral nucleoprotein. Cellular immune responses are essential for recovery, as demonstrated by elevated Th1-dependent plasma interferon-gamma levels during the acute phase and subsequent elevation of Th2-dependent interleukin 4, interleukin 10, and interleukin 13 levels.[9]
Lymph node biopsy will show the characteristic Warthin-Finkeldey giant cells (fused lymphocytes) in a background of paracortical hyperplasia. [10]
The measles virus is known to induce immunosuppression that can last for weeks to months, even years. This causes increased susceptibility to secondary bacterial and other infections. While the mechanisms causing this phenomenon are unclear, it is hypothesized that measles infection induces the proliferation of measles-specific lymphocytes that replace the previously established memory cells causing "immune amnesia." This results in enhanced susceptibility of the host to secondary infections, leading to most of the morbidity and mortality associated with measles. The neutralizing IgG antibodies against hemagglutinin are responsible for lifelong immunity as they block host cell receptors from binding to the virus.
History and Physical
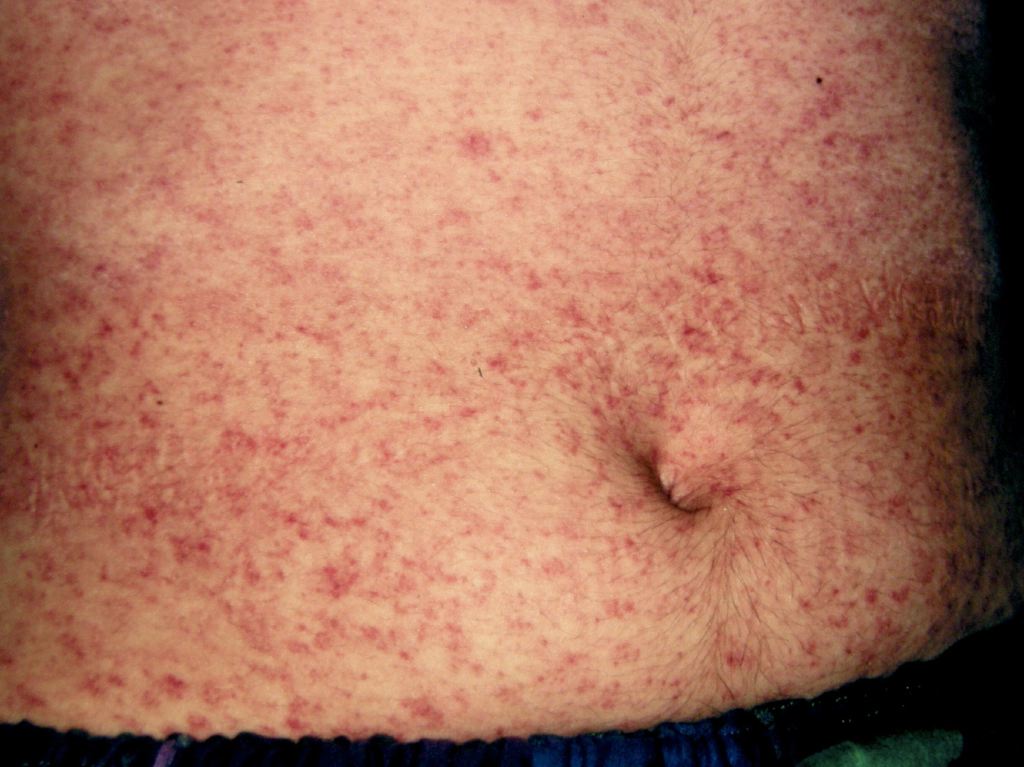
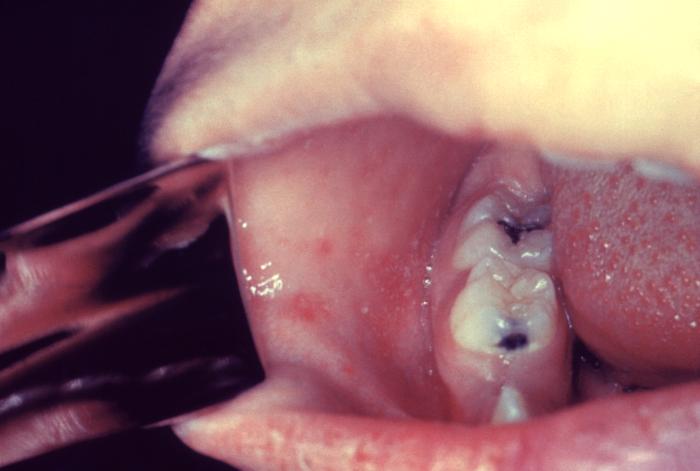
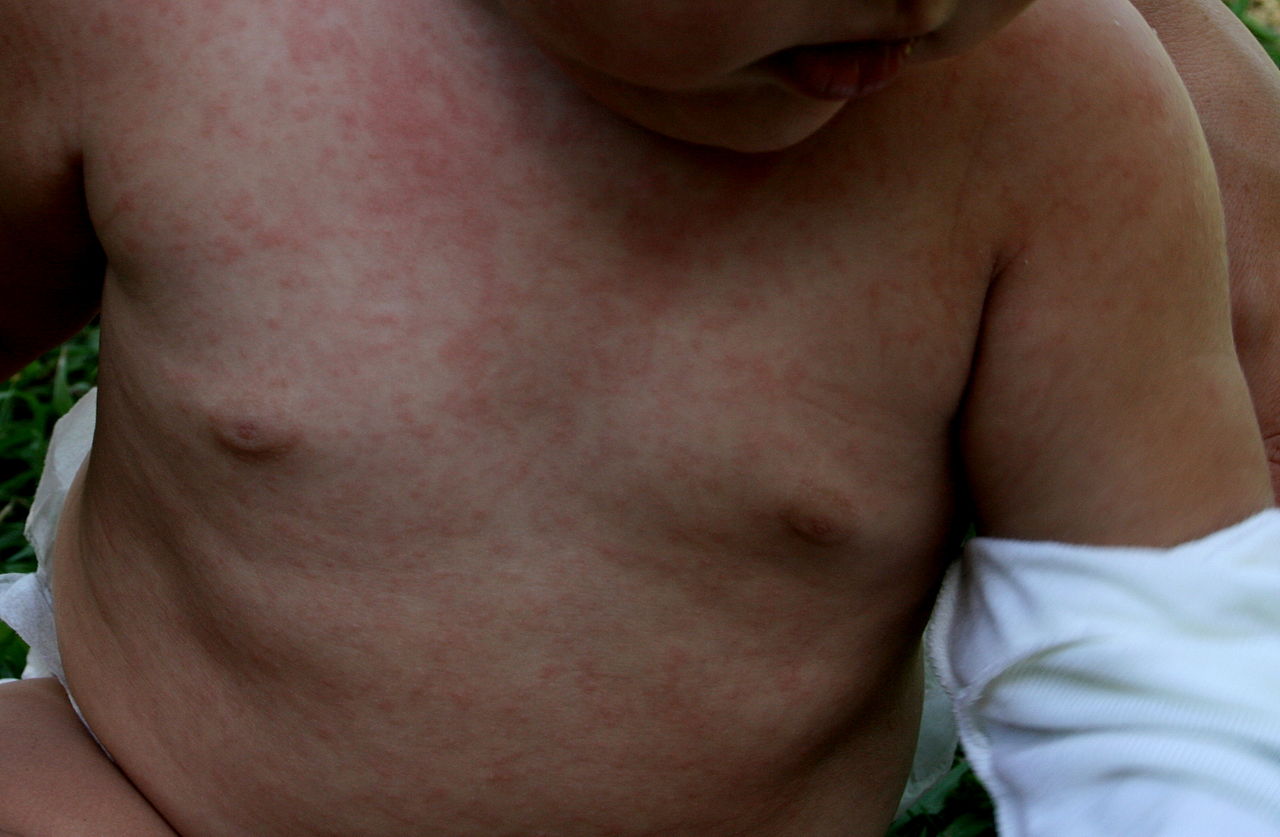
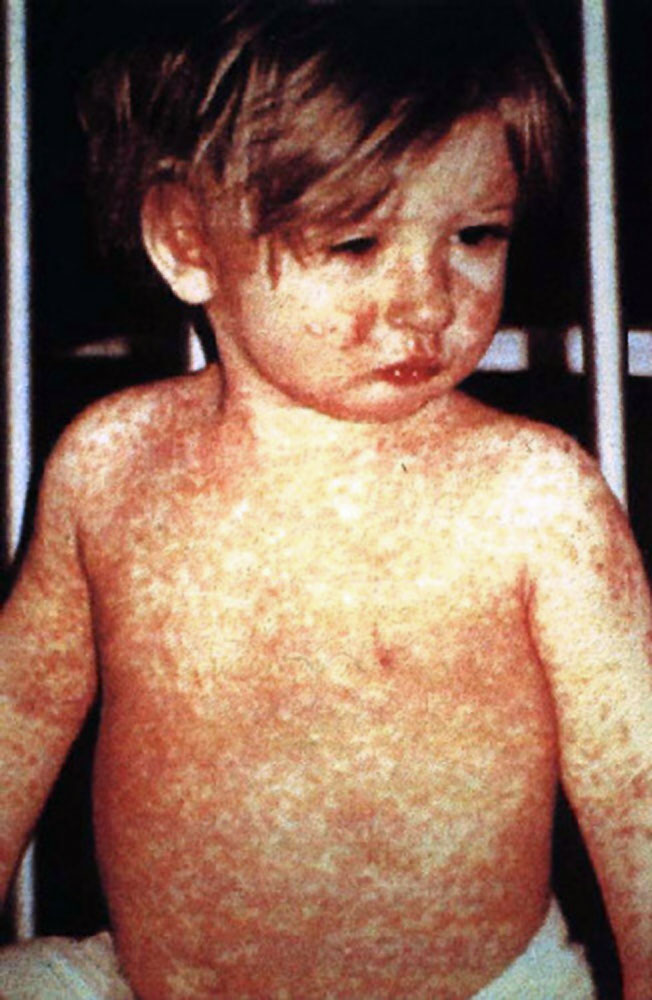
The WHO clinical case definition of measles is "any person with fever, generalized maculopapular rash, cough, coryza, or conjunctivitis." Measles is an acute febrile exanthema that is characterized by the three “Cs”: cough, coryza, and conjunctivitis. Koplik spots, small white papules on the buccal mucosa, are pathognomonic for measles and appear a day or two before the rash, although they are not always seen. The fever precedes the rash. The rash appears first on the face and spreads caudally to become generalized. Uncomplicated measles typically resolves a week after the rash onset.[11]
Evaluation
The diagnosis of measles hinges on a high clinical suspicion, especially when evaluating children with febrile illness and a maculopapular rash. A complete blood count may show leukopenia, particularly lymphopenia, and thrombocytopenia. Electrolyte abnormalities may be detected in children with poor intake or diarrhea. Identification of measles-virus-specific IgM antibodies in serum or plasma confirms the diagnosis, although it may be a false negative in up to 25% of cases when done early (less than 3 days of rash onset.) These antibodies usually peak within 1 to 3 weeks after the onset of a rash and become undetectable by 4 to 8 weeks. The gold standard test is the plaque reduction neutralization assay which has the highest sensitivity. Measle virus can be cultured from nasopharyngeal secretions, but this is labor intensive and not practical.[12]
In current clinical practice, polymerase chain reaction detection of viral ribonucleic acid from throat, nasal, nasopharyngeal, and urine samples is most often performed, with sensitivity approaching 100%.
Treatment / Management
There is no specific antiviral therapy for measles; treatment is primarily supportive. Control of fever, prevention, and correction of dehydration, and infection control measures including appropriate isolation form the mainstay of therapy.[13]
The WHO recommends the administration of daily doses of vitamin A for 2 days and more days for malnourished children. Measles complications should be identified early and appropriate therapy initiated.[14]
Differential Diagnosis
- Pediatric enteroviral infections
- Pediatric toxic shock syndrome
Prognosis
While many will recover from measles with no complications, there is a risk of a poor prognosis. The more common complications due to infection with measles include otitis media and diarrhea. The otitis may lead to hearing loss. Those more likely to have severe complications include infants and children under the age of five years, adults over the age of twenty, pregnant women, and those who are immunocompromised. Encephalitis may occur in 1 out of every 1000 infected children, and 1-2 of all infected children will die of neurologic or respiratory complications from measles.
Complications
Complications of measles occur most commonly in young infants, pregnant women, and malnourished or immunocompromised children. The most common complication is pneumonia which can be due to the measles virus itself (Hecht giant cell pneumonia) or a secondary bacterial infection. Other complications include croup, otitis media, and diarrhea from secondary infections. Pregnant women with measles are at increased risk for maternal death, spontaneous abortion, intrauterine fetal death, and low birth-weight infants. Measles keratoconjunctivitis occurs mostly in children with vitamin A deficiency and can lead to blindness. Central nervous system complications include acute disseminated encephalomyelitis (ADEM), measles inclusion body encephalitis (MIBE), and subacute sclerosing panencephalitis (SSPE).[15] ADEM is an autoimmune demyelinating disease that occurs within days to weeks.[16] MIBE is thought to be a progressive brain infection in patients with impaired cellular immunity occurring within months of the initial infection. SSPE is a progressive neurological disease that presents 5 to 10 years after the acute illness and is thought to be caused by an abnormal host response to the production of mutated virions.[17] It occurs mostly among children that developed measles before 2 years of age and manifest with seizures and progressive loss of cognitive and motor function.
Pearls and Other Issues
Measles is a preventable disease due to the availability of a safe, inexpensive, and effective vaccine. The vaccine is a live attenuated measles strain that is used either as a single component or as a combination vaccine (MMR, MMR-V). The WHO recommends two doses of measles vaccination beginning at age 9 to 12 months and 15 to 18 months in countries where incidence and mortality are still high in the first year of life. In the United States and other developed countries, the first vaccine is given at 12 to 15 months and the second at 4 to 5 years. The WHOs Global Vaccine Action Plan has targeted measles and rubella for elimination in five WHO Regions by 2020. To eliminate measles, vaccination rates of the population must be in the 93% to 95% range.
A widely discredited and withdrawn Lancet article in 1998 created considerable misinformation, purporting a link between the MMR vaccine and autism that led to declining immunization rates, particularly in the United Kingdom and the United States. Many subsequent studies have debunked this myth.
Enhancing Healthcare Team Outcomes
Measles is a preventable infection, and hence all healthcare workers, including nurses and pharmacists should educate patients on the importance of vaccination. Patients should be informed that the adverse effects of the measles vaccination are rare and minor; without vaccination, there is a high risk of transmitting the infection to others and inducing serious neurological complications.