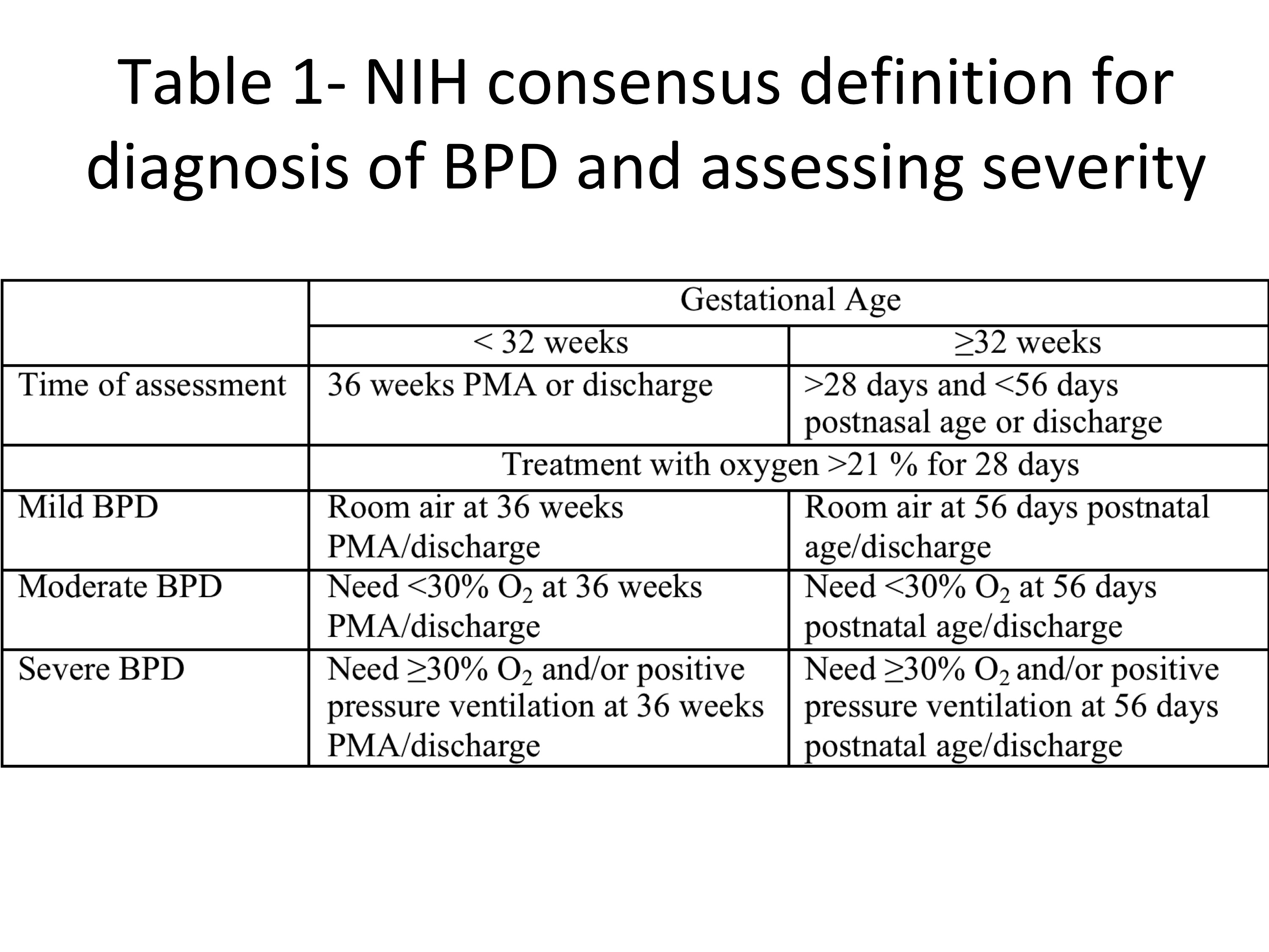
[1]
Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. The New England journal of medicine. 1967 Feb 16:276(7):357-68
[PubMed PMID: 5334613]
[3]
Balany J, Bhandari V. Understanding the Impact of Infection, Inflammation, and Their Persistence in the Pathogenesis of Bronchopulmonary Dysplasia. Frontiers in medicine. 2015:2():90. doi: 10.3389/fmed.2015.00090. Epub 2015 Dec 21
[PubMed PMID: 26734611]
Level 3 (low-level) evidence
[4]
Jensen EA, Schmidt B. Epidemiology of bronchopulmonary dysplasia. Birth defects research. Part A, Clinical and molecular teratology. 2014 Mar:100(3):145-57. doi: 10.1002/bdra.23235. Epub 2014 Mar 17
[PubMed PMID: 24639412]
[5]
Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, Kennedy KA, Poindexter BB, Finer NN, Ehrenkranz RA, Duara S, Sánchez PJ, O'Shea TM, Goldberg RN, Van Meurs KP, Faix RG, Phelps DL, Frantz ID 3rd, Watterberg KL, Saha S, Das A, Higgins RD, Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010 Sep:126(3):443-56. doi: 10.1542/peds.2009-2959. Epub 2010 Aug 23
[PubMed PMID: 20732945]
[6]
Morty RE. Recent advances in the pathogenesis of BPD. Seminars in perinatology. 2018 Nov:42(7):404-412. doi: 10.1053/j.semperi.2018.09.001. Epub 2018 Oct 2
[PubMed PMID: 30384986]
Level 3 (low-level) evidence
[7]
Coalson JJ. Pathology of new bronchopulmonary dysplasia. Seminars in neonatology : SN. 2003 Feb:8(1):73-81
[PubMed PMID: 12667832]
[8]
Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, Wrage LA, Poole K, National Institutes of Child Health and Human Development Neonatal Research Network. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005 Dec:116(6):1353-60
[PubMed PMID: 16322158]
Level 3 (low-level) evidence
[9]
Higgins RD, Jobe AH, Koso-Thomas M, Bancalari E, Viscardi RM, Hartert TV, Ryan RM, Kallapur SG, Steinhorn RH, Konduri GG, Davis SD, Thebaud B, Clyman RI, Collaco JM, Martin CR, Woods JC, Finer NN, Raju TNK. Bronchopulmonary Dysplasia: Executive Summary of a Workshop. The Journal of pediatrics. 2018 Jun:197():300-308. doi: 10.1016/j.jpeds.2018.01.043. Epub 2018 Mar 16
[PubMed PMID: 29551318]
[10]
Ibrahim J, Bhandari V. The definition of bronchopulmonary dysplasia: an evolving dilemma. Pediatric research. 2018 Nov:84(5):586-588. doi: 10.1038/s41390-018-0167-9. Epub 2018 Sep 4
[PubMed PMID: 30188504]
[11]
Arigliani M, Spinelli AM, Liguoro I, Cogo P. Nutrition and Lung Growth. Nutrients. 2018 Jul 18:10(7):. doi: 10.3390/nu10070919. Epub 2018 Jul 18
[PubMed PMID: 30021997]
[12]
Kim LY, McGrath-Morrow SA, Collaco JM. Impact of breast milk on respiratory outcomes in infants with bronchopulmonary dysplasia. Pediatric pulmonology. 2019 Mar:54(3):313-318. doi: 10.1002/ppul.24228. Epub 2019 Jan 4
[PubMed PMID: 30609293]
[13]
Ambalavanan N, Wu TJ, Tyson JE, Kennedy KA, Roane C, Carlo WA. A comparison of three vitamin A dosing regimens in extremely-low-birth-weight infants. The Journal of pediatrics. 2003 Jun:142(6):656-61
[PubMed PMID: 12838194]
[14]
Pasha AB, Chen XQ, Zhou GP. Bronchopulmonary dysplasia: Pathogenesis and treatment. Experimental and therapeutic medicine. 2018 Dec:16(6):4315-4321. doi: 10.3892/etm.2018.6780. Epub 2018 Sep 19
[PubMed PMID: 30542380]
[15]
Bhandari V. The potential of non-invasive ventilation to decrease BPD. Seminars in perinatology. 2013 Apr:37(2):108-14. doi: 10.1053/j.semperi.2013.01.007. Epub
[PubMed PMID: 23582965]
[16]
Dumpa V, Bhandari V. Surfactant, steroids and non-invasive ventilation in the prevention of BPD. Seminars in perinatology. 2018 Nov:42(7):444-452. doi: 10.1053/j.semperi.2018.09.006. Epub 2018 Oct 2
[PubMed PMID: 30343941]
[17]
Committee on Fetus and Newborn. Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics. 2002 Feb:109(2):330-8
[PubMed PMID: 11826218]
[18]
Baud O, Maury L, Lebail F, Ramful D, El Moussawi F, Nicaise C, Zupan-Simunek V, Coursol A, Beuchée A, Bolot P, Andrini P, Mohamed D, Alberti C, PREMILOC trial study group. Effect of early low-dose hydrocortisone on survival without bronchopulmonary dysplasia in extremely preterm infants (PREMILOC): a double-blind, placebo-controlled, multicentre, randomised trial. Lancet (London, England). 2016 Apr 30:387(10030):1827-36. doi: 10.1016/S0140-6736(16)00202-6. Epub 2016 Feb 23
[PubMed PMID: 26916176]
Level 1 (high-level) evidence
[19]
Doyle LW, Davis PG, Morley CJ, McPhee A, Carlin JB, DART Study Investigators. Low-dose dexamethasone facilitates extubation among chronically ventilator-dependent infants: a multicenter, international, randomized, controlled trial. Pediatrics. 2006 Jan:117(1):75-83
[PubMed PMID: 16396863]
Level 1 (high-level) evidence
[20]
Shah VS, Ohlsson A, Halliday HL, Dunn M. Early administration of inhaled corticosteroids for preventing chronic lung disease in very low birth weight preterm neonates. The Cochrane database of systematic reviews. 2017 Jan 4:1(1):CD001969. doi: 10.1002/14651858.CD001969.pub4. Epub 2017 Jan 4
[PubMed PMID: 28052185]
Level 1 (high-level) evidence
[21]
Stewart A, Brion LP, Ambrosio-Perez I. Diuretics acting on the distal renal tubule for preterm infants with (or developing) chronic lung disease. The Cochrane database of systematic reviews. 2011 Sep 7:2011(9):CD001817. doi: 10.1002/14651858.CD001817.pub2. Epub 2011 Sep 7
[PubMed PMID: 21901679]
Level 1 (high-level) evidence
[22]
Sosulski R, Abbasi S, Bhutani VK, Fox WW. Physiologic effects of terbutaline on pulmonary function of infants with bronchopulmonary dysplasia. Pediatric pulmonology. 1986 Sep-Oct:2(5):269-73
[PubMed PMID: 3774383]
[23]
Anderson PJ, Doyle LW. Neurodevelopmental outcome of bronchopulmonary dysplasia. Seminars in perinatology. 2006 Aug:30(4):227-32
[PubMed PMID: 16860163]
[24]
Bhandari A, Bhandari V. Pitfalls, problems, and progress in bronchopulmonary dysplasia. Pediatrics. 2009 Jun:123(6):1562-73. doi: 10.1542/peds.2008-1962. Epub
[PubMed PMID: 19482769]