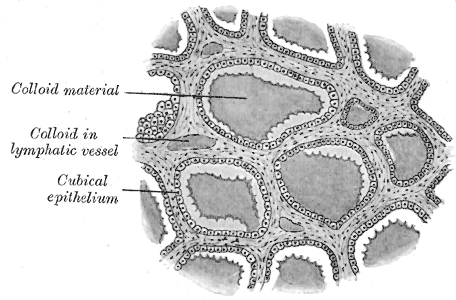
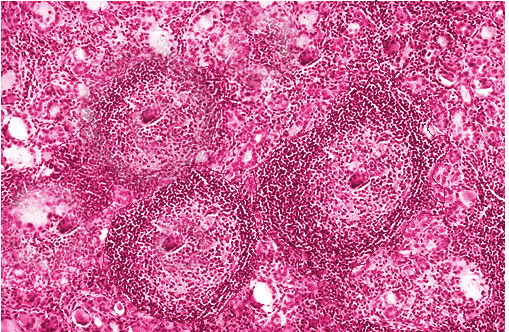
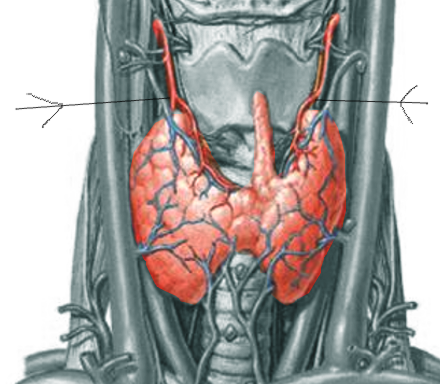
[1]
Chaudhary P, Singh Z, Khullar M, Arora K. Levator glandulae thyroideae, a fibromusculoglandular band with absence of pyramidal lobe and its innervation: a case report. Journal of clinical and diagnostic research : JCDR. 2013 Jul:7(7):1421-4. doi: 10.7860/JCDR/2013/6144.3186. Epub 2013 Jul 1
[PubMed PMID: 23998080]
Level 3 (low-level) evidence
[2]
Esen K, Ozgur A, Balci Y, Tok S, Kara E. Variations in the origins of the thyroid arteries on CT angiography. Japanese journal of radiology. 2018 Feb:36(2):96-102. doi: 10.1007/s11604-017-0710-3. Epub 2017 Dec 4
[PubMed PMID: 29204764]
[3]
Giulea C, Enciu O, Toma EA, Calu V, Miron A. The Tubercle of Zuckerkandl is Associated with Increased Rates of Transient Postoperative Hypocalcemia and Recurrent Laryngeal Nerve Palsy After Total Thyroidectomy. Chirurgia (Bucharest, Romania : 1990). 2019 Sept-Oct:114(5):579-585. doi: 10.21614/chirurgia.114.5.579. Epub
[PubMed PMID: 31670633]
[5]
Rykova Y, Shuper S, Shcherbakovsky M, Kikinchuk V, Peshenko A. [MORPHOLOGICAL CHARACTERISTICS OF THE THYROID GLAND OF MATURE RATS IN MODERATE DEGREE CHRONIC HYPERTHERMIA]. Georgian medical news. 2019 Jul-Aug:(292-293):75-81
[PubMed PMID: 31560668]
[6]
Hofstee P, Bartho LA, McKeating DR, Radenkovic F, McEnroe G, Fisher JJ, Holland OJ, Vanderlelie JJ, Perkins AV, Cuffe JSM. Maternal selenium deficiency during pregnancy in mice increases thyroid hormone concentrations, alters placental function and reduces fetal growth. The Journal of physiology. 2019 Dec:597(23):5597-5617. doi: 10.1113/JP278473. Epub 2019 Oct 30
[PubMed PMID: 31562642]
[7]
Talat A, Khan AA, Nasreen S, Wass JA. Thyroid Screening During Early Pregnancy and the Need for Trimester Specific Reference Ranges: A Cross-Sectional Study in Lahore, Pakistan. Cureus. 2019 Sep 15:11(9):e5661. doi: 10.7759/cureus.5661. Epub 2019 Sep 15
[PubMed PMID: 31720137]
Level 2 (mid-level) evidence
[8]
Zhu B, Zhao G, Yang L, Zhou B. Tetrabromobisphenol A caused neurodevelopmental toxicity via disrupting thyroid hormones in zebrafish larvae. Chemosphere. 2018 Apr:197():353-361. doi: 10.1016/j.chemosphere.2018.01.080. Epub
[PubMed PMID: 29407805]
[9]
Delitala AP, Scuteri A, Maioli M, Mangatia P, Vilardi L, Erre GL. Subclinical hypothyroidism and cardiovascular risk factors. Minerva medica. 2019 Dec:110(6):530-545. doi: 10.23736/S0026-4806.19.06292-X. Epub 2019 Nov 11
[PubMed PMID: 31726814]
[10]
Tost M,Monreal JA,Armario A,Barbero JD,Cobo J,García-Rizo C,Bioque M,Usall J,Huerta-Ramos E,Soria V,Labad J, Targeting Hormones for Improving Cognition in Major Mood Disorders and Schizophrenia: Thyroid Hormones and Prolactin. Clinical drug investigation. 2019 Oct 14;
[PubMed PMID: 31612424]
[11]
Lee JH, Kwon OD, Ahn SH, Choi KH, Park JH, Lee S, Choi BK, Jung KY. Reduction of gastrointestinal motility by unilateral thyroparathyroidectomy plus subdiaphragmatic vagotomy in rats. World journal of gastroenterology. 2012 Sep 7:18(33):4570-7. doi: 10.3748/wjg.v18.i33.4570. Epub
[PubMed PMID: 22969231]
[12]
Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, Jasim S, Dean DS, Gharib H. Fine-Needle Aspiration of the Thyroid Gland. Endotext. 2000:():
[PubMed PMID: 25905400]
[13]
Keenan DM, Pichler Hefti J, Veldhuis JD, Von Wolff M. Regulation and adaptation of endocrine axes at high altitude. American journal of physiology. Endocrinology and metabolism. 2020 Feb 1:318(2):E297-E309. doi: 10.1152/ajpendo.00243.2019. Epub 2019 Nov 26
[PubMed PMID: 31770013]
[14]
Bertoni APS,de Campos RP,Tsao M,Braganhol E,Furlanetto TW,Wink MR, Extracellular ATP is Differentially Metabolized on Papillary Thyroid Carcinoma Cells Surface in Comparison to Normal Cells. Cancer microenvironment : official journal of the International Cancer Microenvironment Society. 2018 Jun
[PubMed PMID: 29455338]
[15]
Nonaka D. A study of FoxA1 expression in thyroid tumors. Human pathology. 2017 Jul:65():217-224. doi: 10.1016/j.humpath.2017.05.007. Epub 2017 May 22
[PubMed PMID: 28546130]
[16]
Dunđerović D, Lipkovski JM, Boričic I, Soldatović I, Božic V, Cvejić D, Tatić S. Defining the value of CD56, CK19, Galectin 3 and HBME-1 in diagnosis of follicular cell derived lesions of thyroid with systematic review of literature. Diagnostic pathology. 2015 Oct 26:10():196. doi: 10.1186/s13000-015-0428-4. Epub 2015 Oct 26
[PubMed PMID: 26503236]
Level 1 (high-level) evidence
[17]
Wong YP, Affandi KA, Tan GC, Muhammad R. Metastasis within a metastasis to the thyroid: A rare phenomenon. Indian journal of pathology & microbiology. 2017 Jul-Sep:60(3):430-432. doi: 10.4103/IJPM.IJPM_287_16. Epub
[PubMed PMID: 28937391]
[18]
Naoum GE,Morkos M,Kim B,Arafat W, Novel targeted therapies and immunotherapy for advanced thyroid cancers. Molecular cancer. 2018 Feb 19
[PubMed PMID: 29455653]
[19]
Kalfert D, Ludvikova M, Kholova I, Ludvik J, Topolocan O, Plzak J. Combined use of galectin-3 and thyroid peroxidase improves the differential diagnosis of thyroid tumors. Neoplasma. 2020 Jan:67(1):164-170. doi: 10.4149/neo_2019_190128N86. Epub 2019 Nov 18
[PubMed PMID: 31777257]
[20]
Lee J, Yi S, Kang YE, Kim HW, Joung KH, Sul HJ, Kim KS, Shong M. Morphological and Functional Changes in the Thyroid Follicles of the Aged Murine and Humans. Journal of pathology and translational medicine. 2016 Nov:50(6):426-435
[PubMed PMID: 27737529]
[21]
Petrova I, Mitevska E, Gerasimovska Z, Milenkova L, Kostovska N. Histological structure of the thyroid gland in apolipoprotein E deficient female mice after levothyroxine application. Prilozi (Makedonska akademija na naukite i umetnostite. Oddelenie za medicinski nauki). 2014:35(3):135-40
[PubMed PMID: 25725701]
[22]
Shoyele O,Bacus B,Haddad L,Li Y,Shidham V, Lymphoproliferative process with reactive follicular cells in thyroid fine-needle aspiration: A few simple but important diagnostic pearls. CytoJournal. 2019;
[PubMed PMID: 31741667]
[23]
Ibhazehiebo K, Koibuchi N. Temporal effects of thyroid hormone (TH) and decabrominated diphenyl ether (BDE209) on Purkinje cell dendrite arborization. Nigerian journal of physiological sciences : official publication of the Physiological Society of Nigeria. 2012 Jun 7:27(1):11-7
[PubMed PMID: 23235302]
[24]
Takizawa T, Yamamoto M, Arishima K, Kusanagi M, Somiya H, Eguchi Y. An electron microscopic study on follicular formation and TSH sensitivity of the fetal rat thyroid gland in organ culture. The Journal of veterinary medical science. 1993 Feb:55(1):157-60
[PubMed PMID: 8461414]
[25]
Rajkovic V, Matavulj M, Johansson O. Light and electron microscopic study of the thyroid gland in rats exposed to power-frequency electromagnetic fields. The Journal of experimental biology. 2006 Sep:209(Pt 17):3322-8
[PubMed PMID: 16916968]
[26]
Shah JP. Thyroid carcinoma: epidemiology, histology, and diagnosis. Clinical advances in hematology & oncology : H&O. 2015 Apr:13(4 Suppl 4):3-6
[PubMed PMID: 26430868]
Level 3 (low-level) evidence
[28]
Felsenfeld AJ, Levine BS. Calcitonin, the forgotten hormone: does it deserve to be forgotten? Clinical kidney journal. 2015 Apr:8(2):180-7. doi: 10.1093/ckj/sfv011. Epub 2015 Mar 20
[PubMed PMID: 25815174]
[30]
Kameda Y. Electron microscopic studies on the parafollicular cells and parafollicular cell complexes in the dog. Archivum histologicum Japonicum = Nihon soshikigaku kiroku. 1973 Dec:36(2):89-105
[PubMed PMID: 4360770]
[31]
Yildirim D, Alis D, Bakir A, Ustabasioglu FE, Samanci C, Colakoglu B. Evaluation of parenchymal thyroid diseases with multiparametric ultrasonography. The Indian journal of radiology & imaging. 2017 Oct-Dec:27(4):463-469. doi: 10.4103/ijri.IJRI_409_16. Epub
[PubMed PMID: 29379243]
[32]
Albasri A, Sawaf Z, Hussainy AS, Alhujaily A. Histopathological patterns of thyroid disease in Al-Madinah region of Saudi Arabia. Asian Pacific journal of cancer prevention : APJCP. 2014:15(14):5565-70
[PubMed PMID: 25081665]
[33]
Qureshi IA, Khabaz MN, Baig M, Begum B, Abdelrehaman AS, Hussain MB. Histopathological findings in goiter: A review of 624 thyroidectomies. Neuro endocrinology letters. 2015:36(1):48-52
[PubMed PMID: 25789588]
Level 3 (low-level) evidence
[34]
DeLellis RA. Orphan Annie eye nuclei: a historical note. The American journal of surgical pathology. 1993 Oct:17(10):1067-8
[PubMed PMID: 8372945]
[35]
Bavle RM. Orphan annie-eye nuclei. Journal of oral and maxillofacial pathology : JOMFP. 2013 May:17(2):154-5. doi: 10.4103/0973-029X.119737. Epub
[PubMed PMID: 24250070]
[36]
Das DK. Psammoma body: a product of dystrophic calcification or of a biologically active process that aims at limiting the growth and spread of tumor? Diagnostic cytopathology. 2009 Jul:37(7):534-41. doi: 10.1002/dc.21081. Epub
[PubMed PMID: 19373908]
[37]
Pyo JS, Kang G, Kim DH, Park C, Kim JH, Sohn JH. The prognostic relevance of psammoma bodies and ultrasonographic intratumoral calcifications in papillary thyroid carcinoma. World journal of surgery. 2013 Oct:37(10):2330-5. doi: 10.1007/s00268-013-2107-5. Epub
[PubMed PMID: 23716027]
[38]
Chaudhary M, Baisakhiya N, Singh G. Clinicopathological and Radiological Study of Thyroid Swelling. Indian journal of otolaryngology and head and neck surgery : official publication of the Association of Otolaryngologists of India. 2019 Oct:71(Suppl 1):893-904. doi: 10.1007/s12070-019-01616-y. Epub 2019 Feb 8
[PubMed PMID: 31742091]
[39]
Gurleyik E. Recurrent Goiter Presented with Marine-Lenhart Syndrome 27 Years After Initial Surgery. Cureus. 2019 Sep 26:11(9):e5768. doi: 10.7759/cureus.5768. Epub 2019 Sep 26
[PubMed PMID: 31723527]