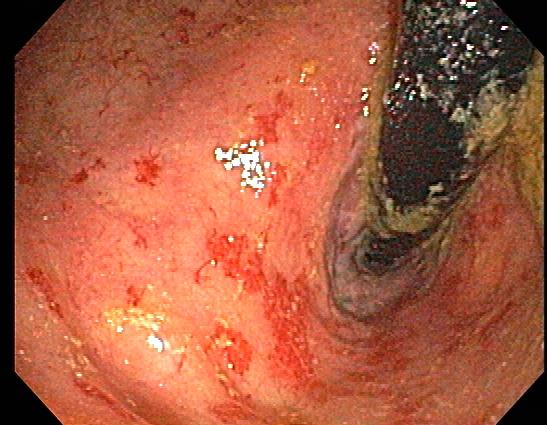
[1]
Ballas LK, Elkin EB, Schrag D, Minsky BD, Bach PB. Radiation therapy facilities in the United States. International journal of radiation oncology, biology, physics. 2006 Nov 15:66(4):1204-11
[PubMed PMID: 17145535]
[2]
Michalski JM, Gay H, Jackson A, Tucker SL, Deasy JO. Radiation dose-volume effects in radiation-induced rectal injury. International journal of radiation oncology, biology, physics. 2010 Mar 1:76(3 Suppl):S123-9. doi: 10.1016/j.ijrobp.2009.03.078. Epub
[PubMed PMID: 20171506]
[3]
Kerns SL, Fachal L, Dorling L, Barnett GC, Baran A, Peterson DR, Hollenberg M, Hao K, Narzo AD, Ahsen ME, Pandey G, Bentzen SM, Janelsins M, Elliott RM, Pharoah PDP, Burnet NG, Dearnaley DP, Gulliford SL, Hall E, Sydes MR, Aguado-Barrera ME, Gómez-Caamaño A, Carballo AM, Peleteiro P, Lobato-Busto R, Stock R, Stone NN, Ostrer H, Usmani N, Singhal S, Tsuji H, Imai T, Saito S, Eeles R, DeRuyck K, Parliament M, Dunning AM, Vega A, Rosenstein BS, West CML. Radiogenomics Consortium Genome-Wide Association Study Meta-Analysis of Late Toxicity After Prostate Cancer Radiotherapy. Journal of the National Cancer Institute. 2020 Feb 1:112(2):179-190. doi: 10.1093/jnci/djz075. Epub
[PubMed PMID: 31095341]
Level 1 (high-level) evidence
[4]
Willett CG, Ooi CJ, Zietman AL, Menon V, Goldberg S, Sands BE, Podolsky DK. Acute and late toxicity of patients with inflammatory bowel disease undergoing irradiation for abdominal and pelvic neoplasms. International journal of radiation oncology, biology, physics. 2000 Mar 1:46(4):995-8
[PubMed PMID: 10705022]
[5]
Hoffman R, Welton ML, Klencke B, Weinberg V, Krieg R. The significance of pretreatment CD4 count on the outcome and treatment tolerance of HIV-positive patients with anal cancer. International journal of radiation oncology, biology, physics. 1999 Apr 1:44(1):127-31
[PubMed PMID: 10219805]
[6]
Otchy DP, Nelson H. Radiation injuries of the colon and rectum. The Surgical clinics of North America. 1993 Oct:73(5):1017-35
[PubMed PMID: 8378826]
[7]
Gilinsky NH, Burns DG, Barbezat GO, Levin W, Myers HS, Marks IN. The natural history of radiation-induced proctosigmoiditis: an analysis of 88 patients. The Quarterly journal of medicine. 1983 Winter:52(205):40-53
[PubMed PMID: 6603628]
[8]
Palmer JA, Bush RS. Radiation injuries to the bowel associated with the treatment of carcinoma of the cervix. Surgery. 1976 Oct:80(4):458-64
[PubMed PMID: 968730]
[9]
Hauer-Jensen M, Denham JW, Andreyev HJ. Radiation enteropathy--pathogenesis, treatment and prevention. Nature reviews. Gastroenterology & hepatology. 2014 Aug:11(8):470-9. doi: 10.1038/nrgastro.2014.46. Epub 2014 Apr 1
[PubMed PMID: 24686268]
[10]
Villasanta U. Complications of radiotherapy for carcinoma of the uterine cervix. American journal of obstetrics and gynecology. 1972 Nov 15:114(6):717-26
[PubMed PMID: 4633571]
[11]
Varma JS, Smith AN, Busuttil A. Correlation of clinical and manometric abnormalities of rectal function following chronic radiation injury. The British journal of surgery. 1985 Nov:72(11):875-8
[PubMed PMID: 4063752]
[12]
Sharma B, Gupta M, Sharma R, Gupta A, Sharma N, Sharma M, Sharma V, Vats S, Gupta M, Seam RK. Four percent formalin application for the management of radiation proctitis in carcinoma cervix patients: An effective, safe, and economical practice. Journal of cancer research and therapeutics. 2019 Jan-Mar:15(1):92-95. doi: 10.4103/jcrt.JCRT_393_17. Epub
[PubMed PMID: 30880761]
[13]
Kapp KS, Stuecklschweiger GF, Kapp DS, Poschauko J, Pickel H, Hackl A. Carcinoma of the cervix: analysis of complications after primary external beam radiation and Ir-192 HDR brachytherapy. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 1997 Feb:42(2):143-53
[PubMed PMID: 9106923]
[14]
Ali F, Hu KY. Evaluation and Management of Chronic Radiation Proctitis. Diseases of the colon and rectum. 2020 Mar:63(3):285-287. doi: 10.1097/DCR.0000000000001592. Epub
[PubMed PMID: 32032142]
[15]
Mendelson RM, Nolan DJ. The radiological features of chronic radiation enteritis. Clinical radiology. 1985 Mar:36(2):141-8
[PubMed PMID: 4064491]
[16]
Muecke R, Schomburg L, Glatzel M, Berndt-Skorka R, Baaske D, Reichl B, Buentzel J, Kundt G, Prott FJ, Devries A, Stoll G, Kisters K, Bruns F, Schaefer U, Willich N, Micke O, German Working Group Trace Elements and Electrolytes in Oncology-AKTE. Multicenter, phase 3 trial comparing selenium supplementation with observation in gynecologic radiation oncology. International journal of radiation oncology, biology, physics. 2010 Nov 1:78(3):828-35. doi: 10.1016/j.ijrobp.2009.08.013. Epub 2010 Feb 3
[PubMed PMID: 20133068]
[17]
Zelefsky MJ, Levin EJ, Hunt M, Yamada Y, Shippy AM, Jackson A, Amols HI. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. International journal of radiation oncology, biology, physics. 2008 Mar 15:70(4):1124-9. doi: 10.1016/j.ijrobp.2007.11.044. Epub
[PubMed PMID: 18313526]
[18]
Chopra S, Gupta S, Kannan S, Dora T, Engineer R, Mangaj A, Maheshwari A, Shylasree TS, Ghosh J, Paul SN, Phurailatpam R, Charnalia M, Alone M, Swamidas J, Mahantshetty U, Deodhar K, Kerkar R, Shrivastava SK. Late Toxicity After Adjuvant Conventional Radiation Versus Image-Guided Intensity-Modulated Radiotherapy for Cervical Cancer (PARCER): A Randomized Controlled Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2021 Nov 20:39(33):3682-3692. doi: 10.1200/JCO.20.02530. Epub 2021 Sep 10
[PubMed PMID: 34506246]
Level 1 (high-level) evidence
[19]
Klopp AH, Yeung AR, Deshmukh S, Gil KM, Wenzel L, Westin SN, Gifford K, Gaffney DK, Small W Jr, Thompson S, Doncals DE, Cantuaria GHC, Yaremko BP, Chang A, Kundapur V, Mohan DS, Haas ML, Kim YB, Ferguson CL, Pugh SL, Kachnic LA, Bruner DW. Patient-Reported Toxicity During Pelvic Intensity-Modulated Radiation Therapy: NRG Oncology-RTOG 1203. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018 Aug 20:36(24):2538-2544. doi: 10.1200/JCO.2017.77.4273. Epub 2018 Jul 10
[PubMed PMID: 29989857]
[20]
Pan HY, Jiang J, Hoffman KE, Tang C, Choi SL, Nguyen QN, Frank SJ, Anscher MS, Shih YT, Smith BD. Comparative Toxicities and Cost of Intensity-Modulated Radiotherapy, Proton Radiation, and Stereotactic Body Radiotherapy Among Younger Men With Prostate Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018 Jun 20:36(18):1823-1830. doi: 10.1200/JCO.2017.75.5371. Epub 2018 Mar 21
[PubMed PMID: 29561693]
Level 2 (mid-level) evidence
[21]
Fang P, Mick R, Deville C, Both S, Bekelman JE, Christodouleas JP, Guzzo TJ, Tochner Z, Hahn SM, Vapiwala N. A case-matched study of toxicity outcomes after proton therapy and intensity-modulated radiation therapy for prostate cancer. Cancer. 2015 Apr 1:121(7):1118-27. doi: 10.1002/cncr.29148. Epub 2014 Nov 25
[PubMed PMID: 25423899]
Level 3 (low-level) evidence
[22]
Delobel JB, Gnep K, Ospina JD, Beckendorf V, Chira C, Zhu J, Bossi A, Messai T, Acosta O, Castelli J, de Crevoisier R. Nomogram to predict rectal toxicity following prostate cancer radiotherapy. PloS one. 2017:12(6):e0179845. doi: 10.1371/journal.pone.0179845. Epub 2017 Jun 22
[PubMed PMID: 28640871]
[23]
Mariados N, Sylvester J, Shah D, Karsh L, Hudes R, Beyer D, Kurtzman S, Bogart J, Hsi RA, Kos M, Ellis R, Logsdon M, Zimberg S, Forsythe K, Zhang H, Soffen E, Francke P, Mantz C, Rossi P, DeWeese T, Hamstra DA, Bosch W, Gay H, Michalski J. Hydrogel Spacer Prospective Multicenter Randomized Controlled Pivotal Trial: Dosimetric and Clinical Effects of Perirectal Spacer Application in Men Undergoing Prostate Image Guided Intensity Modulated Radiation Therapy. International journal of radiation oncology, biology, physics. 2015 Aug 1:92(5):971-977. doi: 10.1016/j.ijrobp.2015.04.030. Epub 2015 Apr 23
[PubMed PMID: 26054865]
Level 1 (high-level) evidence
[24]
Vernia P, Fracasso PL, Casale V, Villotti G, Marcheggiano A, Stigliano V, Pinnaro P, Bagnardi V, Caprilli R. Topical butyrate for acute radiation proctitis: randomised, crossover trial. Lancet (London, England). 2000 Oct 7:356(9237):1232-5
[PubMed PMID: 11072942]
Level 1 (high-level) evidence
[25]
McElvanna K, Wilson A, Irwin T. Sucralfate paste enema: a new method of topical treatment for haemorrhagic radiation proctitis. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. 2014 Apr:16(4):281-4. doi: 10.1111/codi.12507. Epub
[PubMed PMID: 24299100]
[26]
Kochhar R, Patel F, Dhar A, Sharma SC, Ayyagari S, Aggarwal R, Goenka MK, Gupta BD, Mehta SK. Radiation-induced proctosigmoiditis. Prospective, randomized, double-blind controlled trial of oral sulfasalazine plus rectal steroids versus rectal sucralfate. Digestive diseases and sciences. 1991 Jan:36(1):103-7
[PubMed PMID: 1670631]
Level 1 (high-level) evidence
[27]
Sandor Z, Nagata M, Kusstatscher S, Szabo S. Stimulation of mucosal glutathione and angiogenesis: new mechanisms of gastroprotection and ulcer healing by sucralfate. Scandinavian journal of gastroenterology. Supplement. 1995:210():19-21
[PubMed PMID: 8578199]
[28]
Isenberg GA, Goldstein SD, Resnik AM. Formalin therapy for radiation proctitis. JAMA. 1994 Dec 21:272(23):1822
[PubMed PMID: 7990213]
[29]
Dziki Ł, Kujawski R, Mik M, Berut M, Dziki A, Trzciński R. Formalin therapy for hemorrhagic radiation proctitis. Pharmacological reports : PR. 2015 Oct:67(5):896-900. doi: 10.1016/j.pharep.2015.03.006. Epub 2015 Mar 21
[PubMed PMID: 26398382]
[30]
Lucidarme D, Marteau P, Foucault M, Vautrin B, Filoche B. Efficacy and tolerance of mesalazine suppositories vs. hydrocortisone foam in proctitis. Alimentary pharmacology & therapeutics. 1997 Apr:11(2):335-40
[PubMed PMID: 9146772]
[31]
al-Sabbagh R, Sinicrope FA, Sellin JH, Shen Y, Roubein L. Evaluation of short-chain fatty acid enemas: treatment of radiation proctitis. The American journal of gastroenterology. 1996 Sep:91(9):1814-6
[PubMed PMID: 8792704]
[32]
Paquette IM, Vogel JD, Abbas MA, Feingold DL, Steele SR, Clinical Practice Guidelines Committee of The American Society of Colon and Rectal Surgeons. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Treatment of Chronic Radiation Proctitis. Diseases of the colon and rectum. 2018 Oct:61(10):1135-1140. doi: 10.1097/DCR.0000000000001209. Epub
[PubMed PMID: 30192320]
Level 1 (high-level) evidence
[33]
Oscarsson N, Arnell P, Lodding P, Ricksten SE, Seeman-Lodding H. Hyperbaric oxygen treatment in radiation-induced cystitis and proctitis: a prospective cohort study on patient-perceived quality of recovery. International journal of radiation oncology, biology, physics. 2013 Nov 15:87(4):670-5. doi: 10.1016/j.ijrobp.2013.07.039. Epub 2013 Sep 11
[PubMed PMID: 24035333]
Level 2 (mid-level) evidence
[34]
Hoggan BL, Cameron AL. Systematic review of hyperbaric oxygen therapy for the treatment of non-neurological soft tissue radiation-related injuries. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2014 Jun:22(6):1715-26. doi: 10.1007/s00520-014-2198-z. Epub 2014 Mar 29
[PubMed PMID: 24794980]
Level 1 (high-level) evidence
[35]
Clarke RE, Tenorio LM, Hussey JR, Toklu AS, Cone DL, Hinojosa JG, Desai SP, Dominguez Parra L, Rodrigues SD, Long RJ, Walker MB. Hyperbaric oxygen treatment of chronic refractory radiation proctitis: a randomized and controlled double-blind crossover trial with long-term follow-up. International journal of radiation oncology, biology, physics. 2008 Sep 1:72(1):134-143. doi: 10.1016/j.ijrobp.2007.12.048. Epub 2008 Mar 14
[PubMed PMID: 18342453]
Level 1 (high-level) evidence
[36]
Bennett MH, Feldmeier J, Hampson NB, Smee R, Milross C. Hyperbaric oxygen therapy for late radiation tissue injury. The Cochrane database of systematic reviews. 2016 Apr 28:4(4):CD005005. doi: 10.1002/14651858.CD005005.pub4. Epub 2016 Apr 28
[PubMed PMID: 27123955]
Level 1 (high-level) evidence
[37]
Swan MP, Moore GT, Sievert W, Devonshire DA. Efficacy and safety of single-session argon plasma coagulation in the management of chronic radiation proctitis. Gastrointestinal endoscopy. 2010 Jul:72(1):150-4. doi: 10.1016/j.gie.2010.01.065. Epub 2010 May 20
[PubMed PMID: 20493484]
[38]
Jao SW, Beart RW Jr, Gunderson LL. Surgical treatment of radiation injuries of the colon and rectum. American journal of surgery. 1986 Feb:151(2):272-7
[PubMed PMID: 3946764]
[39]
Pricolo VE, Shellito PC. Surgery for radiation injury to the large intestine. Variables influencing outcome. Diseases of the colon and rectum. 1994 Jul:37(7):675-84
[PubMed PMID: 8026234]
[40]
Lev EL, Eller LS, Gejerman G, Lane P, Owen SV, White M, Nganga N. Quality of life of men treated with brachytherapies for prostate cancer. Health and quality of life outcomes. 2004 Jun 15:2():28
[PubMed PMID: 15198803]
Level 2 (mid-level) evidence
[41]
Liauw SL, Sylvester JE, Morris CG, Blasko JC, Grimm PD. Second malignancies after prostate brachytherapy: incidence of bladder and colorectal cancers in patients with 15 years of potential follow-up. International journal of radiation oncology, biology, physics. 2006 Nov 1:66(3):669-73
[PubMed PMID: 16887293]