Continuing Education Activity
Herpes simplex keratitis is a common and potentially blinding condition caused by recurrent corneal infections with the herpes simplex (HSV) virus. The presentations of herpes simplex keratitis vary with conditions such as herpetic dendrites, geographical ulcers, stromal keratitis, disciform keratitis, and neurotrophic keratopathy. Potential ocular complications associated with the disease include reduced corneal sensation, scarring, and blindness.
Herpes simplex keratitis is primarily diagnosed by conducting a thorough clinical evaluation of patients suspected of having the condition, including history-taking and slit-lamp examination. This course explores the complexities surrounding this phenomenon, including the current diagnostic challenges, treatment strategies (eg, topical and systemic antivirals, corticosteroids, and other adjunctive therapies), and recommendations for prophylaxis and management of complications. Healthcare professionals will enhance their ability to provide optimal care for patients with herpes simplex keratitis, ultimately improving patient outcomes and reducing the risk of vision loss associated with this condition.
Objectives:
Determine the pathophysiology of herpes simplex keratitis.
Identify the clinical features of herpes simplex keratitis.
Assess patients for common complications of herpes simplex keratitis.
Apply interprofessional team strategies to improve care coordination and outcomes for patients with herpes simplex keratitis.
Introduction
Herpes simplex keratitis is a common and potentially blinding condition caused by recurrent corneal infections with the herpes simplex virus (HSV). Herpes simplex keratitis remains the leading infectious cause of corneal ulcers and blindness worldwide.[1][2] The most ubiquitous types of HSV are HSV-1 and HSV-2, which affect humans, their only natural hosts. HSV-1 is usually the cause of infections in the oral, labial, and ocular areas; HSV-2 primarily causes lesions in the genital region, although this epidemiology is now changing. Primary infection with HSV occurs after direct contact inoculation of mucosal or skin surface, often subclinical and going unnoticed. After the initial HSV infection, the virus becomes latent, traveling to the dorsal root ganglia and remaining there for the person's lifetime. Subsequent infection is caused by viral reactivation in the affected dermatome.[3]
Herpes simplex keratitis can result from primary HSV ocular infection or recurrent infection. After the initial infection in the ocular area, the virus travels to the trigeminal ganglion via the first branch of the trigeminal nerve (V1), remaining latent. HSV travels back to the cornea when reactivated, inciting an inflammatory response. The conjunctiva, cornea, anterior, iris, lens, vitreous, and retina may be involved. HSV recurrence within the cornea causes herpes simplex keratitis.[3] Manifestations of herpes simplex keratitis include herpetic dendrites, geographical ulcers, stromal keratitis, disciform keratitis, and neurotrophic keratopathy, with herpetic stromal keratitis being the most common. Corneal inflammation can cause reduced corneal sensation, scarring, and blindness. Additionally, HSV infection can result in anterior uveitis, iridocyclitis, complicated cataracts, vitritis, and retinal detachment, which can quickly progress to corneal perforation or blindness if left unchecked.[2]
Initially, herpes simplex keratitis is clinically diagnosed, including a meticulous slit lamp evaluation. Although polymerase chain reaction assay (PCR), enzyme-linked immunosorbent assay, and immunofluorescent antibody assay are additional studies that may be performed for diagnostic confirmation, a viral culture is the gold standard.[4] Treatment with topical antibacterials, antivirals, and systemic antivirals aims to restrict viral replication, reduce lesion severity, and prevent further spread.[4]
Etiology
Herpes simplex keratitis is a common and potentially blinding condition caused by recurrent HSV corneal infections. HSV-1 and HSV-2 belong to the alphaherpesvirinae family, an enveloped virus with a linear, double-stranded DNA genome that affects humans.[5] During primary infection, after inoculation, HSV replicates in the epithelia, residing in the host's dorsal root ganglia for life, establishing latency.[5] Historically, HSV-1 has been known to have a tropism to the orofacial and upper body areas and HSV-2 to the genital and lower body area. This distinction is no longer as clear because both HSV types can be found in either region.[3] In addition to direct primary inoculation, HSV transmission, usually HSV-2, may occur via infected maternal vaginal secretions to the newborn during childbirth resulting in neonatal conjunctivitis.[6]
Primary infection usually manifests in childhood by droplet transmission and less frequently by direct inoculation. Most primary infections by HSV are subclinical and manifest as mild fever, malaise, and upper respiratory tract infection. Primary infection can also result in blepharitis and follicular conjunctivitis, which are mild and self-limited. Primary infection during the first 6 months of life is uncommon due to protection by maternal antibodies. Sometimes severe neonatal systemic disease can occur, which mandates prompt diagnosis, meticulous intervention, and intravenous viral drugs to reduce mortality and disability. The presence of maternal antibodies usually means that a dendritic corneal ulcer may be seen.[7]
Furthermore, HSV transmission is heightened in crowded and poorly hygienic environments.[8] After the initial HSV infection, the virus remains latent in the dorsal root ganglia for the host's lifetime, incorporated in the host DNA, though current treatment modalities can eradicate it. Subsequent viral reactivation causes symptoms within the affected dermatome.[5][9][10] Subclinical reactivation can occur periodically, during which HSV is shed, and patients are contagious.[11] Reactivation can be triggered later in life by various stressors, including fever, trauma, immunosuppression, hormonal change, radiation, or UV exposure.[12]
Primary ocular infection with HSV is rarely clinically apparent. However, when apparent, HSV can manifest as self-limited blepharitis, with vesicular lid lesions or follicular conjunctivitis, leading to keratitis.[13] After the initial infection, the virus remains latent in the trigeminal ganglion via the first branch of the trigeminal nerve (V1). HSV travels back to the cornea when reactivated, inciting an inflammatory response. The main ocular complication of HSV recurrence, keratitis, comprises epithelial, stroma, and, more rarely, endothelial types.[14][15] The cornea, anterior, iris, lens, vitreous, and retina can also be involved. Herpes simplex keratitis within the cornea can have varied manifestations, including herpetic dendrites, geographical ulcers, stromal keratitis, disciform keratitis, and neurotrophic keratopathy, with herpetic stromal keratitis being the most common.[14][15]
Epidemiology
Herpetic eye disease is the most prevalent infectious cause of corneal blindness in developed countries. In the United States, the seroprevalence of HSV-1 is >50%, and for HSV-2, in sexually active individuals, >15%.[16][17][18] Studies estimate that 4.85 billion people worldwide have been infected with HSV-1, and 836 million people older than 15 years with HSV-2.[2] Even though prevalence varies by region, the burden of HSV-2 is in the World Health Organization (WHO) Africa region and the lowest in the WHO Americas region.[2][19] Using 2016 data, James et al estimated that the global prevalence of oral HSV-1 infection was 64% in people aged 0 to 49 years and 13% for HSV-2 in people aged 15 to 49 years.[19]
In 2012, Farooq et al estimated the global annual incidence of herpetic keratitis and new monocular visual impairment cases to be 1.5 million and 40,000, respectively.[9] In developing countries, as many as 60% of corneal ulcers are caused by herpes simplex virus, and the burden of disease estimates that as many as 10 million people worldwide may have ocular disease due to herpes simplex virus infection.[9] Primary ocular infection with HSV-1 may occur at any point in life. Studies showed that the mean age for the first ocular HSV-1 infection is 37.4 years in the United States and 25 years in Britain.[20][21]
Dendritic keratitis showed the most frequent recurrences (56.3%), followed by stromal keratitis (29.5%) and geographical lesions (9.8%).[22] The number of recurrences is related to the number of previous attacks, although recurrence also depends upon other factors (eg, immune stressors). Recurrent infections usually occur in a particular pattern, leading to reactivation of cellular and humoral responses.
After primary infection, the virus migrates to the sensory ganglia of the dermatome, where the disease is localized and remains latent. The reported rate of reactivation of ocular herpes after primary infection is about 10% within the first year and 50% at 10 years. The frequency of recurrences is directly related to the number of previous attacks.[23]
Herpes Simplex Keratitis Risk Factors
Risk factors associated with the severe disease include:
- Frequent recurrent episodes
- Atopic eye disease
- Pediatric age group
- Immunodeficiency or immunosuppression
- Malnourishment
- Alcoholism
- Fever
- Stress
- Malaria
- Inappropriate topical steroids may result in geographical ulceration [24]
Pathophysiology
Generally, to manifest symptoms in the cornea, HSV must bypass host defense mechanisms, including the initial innate immune response and the later adaptive response triggered by exposure to viral antigens. HSV invades the corneal epithelial cells by binding to specific receptors on the host cells. The virus then exploits the host's DNA polymerase to multiply and produce new virus particles, which leave the host cell and spread to adjacent cells.[15] HSV can then migrate along the nerves of the cornea to the trigeminal ganglion, remaining dormant until conditions trigger reactivation, potentially leading to repeated episodes of infection.[25]
Epithelial Keratitis
HSV initiates infection in the corneal epithelium, first appearing as small dot-like keratopathy before merging into larger, branched ulcers known as dendritic keratitis, often mimicking the arrangement of the cornea's subbasal nerve plexus.[14] The virus predominantly replicates at the edge and tips of these dendritic lesions, potentially developing into larger geographic ulcers marked by a significant area of dead cells. Epithelial cell swelling occurs within the first day of infection, leading to the breakdown of the outer corneal layers and cell death. In laboratory settings, HSV infection causes corneal epithelial cells to merge into large, multinucleated structures that mirror the dendritic ulcers seen in patients, aiding in avoiding immune detection.
The infected cells' type I interferon production contains the virus, making the surrounding tissue less susceptible to infection and viral multiplication. Additionally, antibodies specific to HSV in the tears and corneal stroma play a crucial role in curbing the infection's spread within the epithelial layer. Despite these defenses, without proper immune response due to immunodeficiency or misuse of corticosteroids, the virus may breach deeper into the cornea, disrupting the foundational layer and potentially leading to a more severe condition involving the corneal stroma.[5]
Stromal Keratitis
Herpes stromal keratitis is predominantly an immune-mediated inflammatory condition. However, viral entities may remain in both latent and active phases within the corneal stroma or migrate from an initial epithelial infection. Investigations have revealed that in scenarios where mice were infected with a variant of HSV incapable of retrograde movement from the sensory ganglion back to the cornea, herpes stromal keratitis still ensued due to the virus within the corneal tissue.[26] Further research has identified HSV DNA and latency-associated transcripts in the corneas of individuals with a history of HSV keratitis, underlining the potential for viral latency within the cornea. Although penetrating keratoplasty can excise latent corneal HSV infections, such interventions often risk rekindling infections in the corneal transplant.[27]
The pathogenesis of herpes stromal keratitis is intricately linked to the dynamic interactions between HSV-infected corneal cells and the subsequent infiltration of inflammatory cells. Insights into these mechanisms have primarily been gleaned from murine and lagomorph infection models. In humans, herpes stromal keratitis typically manifests as a consequence of recurrent infections, with the dormant virus in the trigeminal ganglia reactivating and migrating anterogradely along corneal nerves to reinfect the cornea.[26] This viral replication activates innate immune pathways, releasing cytokines and chemokines from corneal epithelial and stromal cells. This sequence of events triggers an influx of inflammatory cells, including neutrophils, natural killer cells, dendritic cells, and macrophages, aiming to curtail viral dissemination within the corneal tissue. Dendritic cells are pivotal as primary antigen-presenting cells, engulfing viral particles and infected cells and mobilizing towards draining lymph nodes to initiate an adaptive immune response.[28]
The acute phase of herpes stromal keratitis is marked by a significant infiltration of CD4+ T cells into the cornea around 7 to 21 days postinfection, aligning with the clinical manifestation of the disease. These CD4+ T cells secrete an array of cytokines, such as IFN-γ and IL-17, which are instrumental in recruiting and activating a subsequent influx of neutrophils, believed to exacerbate pathological outcomes.[29] The release of cytokines and proteases by these neutrophils is a crucial driver of the hallmark inflammation and tissue degradation observed in herpes stromal keratitis. The accumulation of these inflammatory cells contributes to corneal opacity, obscuring the visual axis and potentially culminating in blindness.[30]
Endothelitis and Uveitis
HSV endotheliitis is frequently observed alongside stromal keratitis or uveitis, reflecting the complex interplay between these ocular conditions. The presence of HSV DNA within endothelial cells, aqueous humor, and the trabecular meshwork underscores the virus's pervasive nature in individuals with HSV endotheliitis or uveitis. Notably, HSV's invasion into endothelial cells is characterized by a discernible decline in cellular density and the emergence of pleomorphism, highlighting the direct cytopathic effects of the virus on endothelial integrity.[14] Advanced imaging techniques (eg, in vivo confocal microscopy) have further elucidated the pathological alterations associated with HSV endotheliitis. These include the detection of pseudoguttata, indicative of endothelial stress or damage, and the infiltration of inflammatory cells, which contribute to the pathogenesis and symptomatology of the condition. Additionally, an increase in the size of intercellular spaces among endothelial cells has been noted, alongside a general reduction in endothelial cell density. These findings collectively illustrate the detrimental impact of HSV on corneal endothelial health, contributing to the disruption of corneal clarity and function in affected eyes.[31]
Inflammatory Cascade
The elucidation of the immunological mechanisms underpinning herpetic keratitis has predominantly utilized mouse models simulating primary corneal infection, providing valuable insights that parallel the pathogenesis observed in human herpes stromal keratitis. However, a significant distinction in the etiology of herpes stromal keratitis in humans, which typically arises from recurrent infections, often with primary infections originating from sites other than the cornea, should be noted.[15] Despite this, the immunological responses elicited by primary corneal infections in mice display dynamics and outcomes comparable to those observed in humans during the reactivation of latent virus migrating from the trigeminal ganglia to the cornea. In both scenarios, the viral presence in the cornea is effectively eliminated by 7 days postinfection (dpi). This initial phase of the infection activates corneal epithelial cells to release cytokines and chemokines, functioning as early signals that recruit various immune cells to the site of infection. This initiation of the innate immune response represents a critical step in the body's defense mechanism, setting the stage for the subsequent immune-mediated processes that characterize the progression and resolution of herpetic keratitis.[32]
Neutrophils
Neutrophils serve as the primary immunological responders upon infection, converging on the cornea significantly during 2 critical phases of the disease's progression. The initial influx of neutrophils between 2 and 5 dpi coincides with the decline of active viral replication by 5 to 7 dpi, suggesting a pivotal role for neutrophils in eradicating the virus from the corneal tissue. Experimental depletion of neutrophils using an antibody targeting the Gr-1 granulocyte marker resulted in a postponed viral clearance from the cornea, underscoring their contributory role.[33] However, subsequent insights revealed that Gr-1 is expressed in a broader range of cells, not exclusively neutrophils, complicating the interpretation of these findings. Further investigations employing a neutrophil-specific Ly6G antibody in a pulmonary infection model did not significantly impact viral clearance, highlighting the complexity of neutrophil functions and the need for continued research in this domain. Despite these ambiguities, the consensus within the scientific literature leans towards a beneficial role for neutrophils in the initial viral clearance from the cornea. Nonetheless, the precise contribution of neutrophils remains an area ripe for further exploration. Contrarily, the secondary surge of neutrophils aligns with the onset of clinical symptoms and exerts a more deleterious effect on corneal integrity. Beginning around 8 to 9 dpi and escalating through 21 dpi, this wave of neutrophils, presumably recruited and activated by CD4+ T cells, engages in degranulation and releases a suite of inflammatory mediators, proteases, and free radicals, culminating in significant tissue damage characteristic of herpes stromal keratitis. Accounting for 70% to 80% of the leukocytic infiltration at this juncture, neutrophils substantially contribute to the corneal opacity observed in the condition. This dual-phase involvement of neutrophils underscores their complex role in the immune response to herpetic keratitis, necessitating further detailed studies to unravel their precise mechanisms of action.[34]
Dendritic cells, macrophages, and natural killer cells
The traditional belief that the cornea is an immunologically inert tissue has been fundamentally revised in light of contemporary findings, revealing that the cornea harbors a variety of bone marrow-derived leukocytes essential for antigen processing and presentation. Dendritic cells, as critical components of this leukocytic population, are proficient phagocytes that capture foreign antigens, migrate to lymph nodes, and engage T cells through major histocompatibility complex class II (MHC-II) molecules, thereby catalyzing the adaptive immune response.[35] Complementing this mechanism, macrophages and natural killer cells play critical roles within the innate immune system by scavenging pathogens and targeting cells displaying diminished class I MHC molecules, respectively. Research indicates that within the first 24 hours postinfection, both dendritic cells and macrophages proliferate within the central cornea and exhibit enhanced activation, as evidenced by increased expression of MHC-II. Pioneering work by Hu et al demonstrated that the targeted elimination of dendritic cells in mice engineered to express diphtheria toxin receptors under the CD11c promoter exacerbated the severity of keratitis. Conversely, the selective depletion of macrophages via clodronate liposomes did not markedly influence the course of the disease.[36] In a more recent study utilizing mice with a conditional knockout of the autophagy-related gene ATG5 in dendritic cells, researchers discovered that the autophagy pathway in dendritic cells, a process critical for efficient antigen processing and presentation, plays a significant role in the pathogenesis of the disease. Intriguingly, inhibiting dendritic cell autophagy alone reduced disease severity, highlighting the complex interplay between autophagy in dendritic cells and the immune response in herpetic keratitis. These findings underscore the dynamic nature of the corneal immune environment and the pivotal roles played by dendritic cells, macrophages, and natural killer cells in orchestrating innate and adaptive immune responses to infection. The nuanced understanding of these cellular mechanisms opens new avenues for targeted therapeutic interventions to mitigate corneal inflammatory diseases.[37]
Helper T cells
Helper T cells (CD4+ T cells) are recognized as pivotal contributors to the pathogenesis of stromal keratitis, orchestrating the inflammatory response that leads to lesion formation. Initial investigations utilizing athymic mice, which lack T and B lymphocytes, revealed that while these mice are susceptible to HSV-1 corneal infection, they do not exhibit the characteristic lesions associated with stromal keratitis. This underscores the essential role of T cells in disease manifestation. In typical immune responses, CD4+ T cells are recruited to the corneal environment around 7 days postinfection (dpi), with their presence increasing to 21 dpi.[38]
A widely accepted paradigm posits that CD4+ T cells, particularly those polarized towards a Th1 response and producing cytokines such as IL-2 and IFN-γ, are instrumental in mediating the recruitment of neutrophils during the secondary phase of the immune response.[39] Experimental interventions using neutralizing antibodies to block these cytokines have been shown to mitigate the progression of herpes stromal keratitis. Furthermore, advancements in immunology have shed light on the proinflammatory functions of interleukin-17 and Th17 cells within the cornea, highlighting their contribution to the disease's exacerbation. The role of regulatory T cells (Tregs), another subtype of CD4+ T cells, has also been identified in keratitis. These cells possess immunomodulatory functions capable of attenuating the immune response, although their precise mechanisms in keratitis progression remain fully elucidated.[15]
Despite the acknowledged importance of CD4+ T cells in herpes stromal keratitis's immunopathogenesis, the specificity of their antigen recognition is still debatable. Some research suggests these T cells may respond to autoantigens exposed during infection. However, this hypothesis faces skepticism due to the lack of observed cross-reactivity with presumed autoantigens and the absence of a correlation with disease severity, challenging the molecular mimicry theory. An alternative explanation, bystander activation, gained traction following observations that reconstituted athymic mice with T cells nonspecific to viral antigens still developed stromal lesions and failed to clear the infection, leading to encephalitis. This nuanced understanding of CD4+ T cell roles, from Th1 and Th17 cell-mediated inflammation to Treg-mediated immunoregulation, underscores the complexity of immune responses in stromal keratitis. Further research into the antigen specificity of CD4+ T cells and their interactions within the corneal environment is essential for developing targeted therapies for herpes stromal keratitis.[40]
Cytotoxic T cells
The investigation into the roles of various T cell subsets, including cytotoxic T cells (CD8+ T cells), in the context of herpes stromal keratitis, has expanded significantly over recent years, enhancing our comprehension of their contributions to the disease's pathogenesis and protection mechanisms. CD8+ T cells, known for their cytotoxic capabilities, are instrumental in the immune system's defense against viral infections. They achieve this by detecting and eliminating infected cells, facilitated by recognizing viral peptides presented by class I major histocompatibility complex (MHC) molecules.[38]
Recent research underscores the potential role of CD8+ T cells in managing recurrent herpes infections. Studies have illustrated that after initial infection, HSV-1-specific CD8+ T cells are activated and migrate to the trigeminal ganglia, forming associations with neurons and playing a crucial role in the immunological memory against the virus. While some researchers assert the protective role of CD8+ T cells during HSV-1 reactivation, others propose a bystander role, indicating a nuanced understanding of these cells in the disease's pathology.[38] Significant effort has been devoted to delineating the CD8+ T cell receptor repertoire in response to herpes keratitis. Initial findings pointed towards a response predominantly targeting a single epitope on the virus's glycoprotein B (gB). Subsequent research adjusted this view, showing that the immunodominant gB epitope represents about half of the CD8+ T cells response in the trigeminal ganglia, with an array of subdominant epitopes also playing critical roles. This comprehensive understanding of T cell epitope specificity is pivotal for developing targeted HSV-1 vaccines.[41]
Furthermore, investigations into CD8+ T cell subpopulations have revealed that individuals with symptomatic HSV-1 infections exhibit a predominance of less differentiated, monofunctional CD8+ T cells. In contrast, asymptomatic HSV-1 seropositive individuals display more differentiated, multifunctional CD8+ T cells specific to HSV-1 gB. Notably, asymptomatic individuals possessed effector memory CD8+ T cells targeting specific HSV-1 epitopes. A combined therapeutic approach, incorporating a vaccine targeting these epitopes and an adenoviral vector expressing the T cell attractant CXCL10, has shown promise in mitigating virus shedding and reducing the recurrence of herpetic disease.[42] This evolving research landscape into CD8+ T cells, alongside other T cell subsets, is forging new paths in understanding and combating herpes stromal keratitis, highlighting the importance of cellular immunity in viral control and offering novel avenues for therapeutic intervention.
Host Immune Factors
The differential response of individuals to HSV-1 infection, with some patients experiencing recurrent symptomatic episodes and others remaining asymptomatic despite viral shedding, underscores the crucial role of host factors in modulating susceptibility to infection. The dynamic interaction between the pathogen and the host's immune response remains a focal point of investigation.[43]
Toll-like receptors
Toll-like receptors (TLRs) represent a fundamental aspect of the innate immune system, characterized by their ubiquitous presence and capacity to detect specific pathogen-associated molecular patterns (PAMPs). To this end, 13 TLRs have been identified, with 9 common to humans and mice.[44] Each TLR is tailored to recognize distinct PAMPs or ligands. In the context of HSV-1, TLRs 2, 3, and 9 play pivotal roles in identifying viral components; TLR2 interacts with viral glycoproteins at the cellular membrane, whereas TLR3 and TLR9, located within endosomes, are responsive to double-stranded RNA and unmethylated CpG motifs, respectively. The engagement of these TLRs with their respective PAMPs initiates a series of intracellular signaling pathways that culminate in cytokine production, inflammation, and the activation of an innate immune defense.[45] While the activation of TLRs constitutes a critical early mechanism for viral detection and initiation of immune responses, excessive TLR-mediated inflammation can lead to detrimental outcomes, including increased infiltration of the cornea, resulting in opacity and potential vision loss. These outcomes highlight the importance of a balanced immune response, where sufficient viral clearance is achieved without provoking adverse inflammatory reactions that compromise ocular health.[46]
Cytokines and chemokines
Cytokines and chemokines, molecular messengers ranging from approximately 5 to 20 kDa, are pivotal in mediating immune responses to infection and cellular distress. These signaling molecules, originating from a diverse array of cells within the cornea and the broader immune system, engage specific receptors to initiate a spectrum of cellular functions, including activation, differentiation, and migration. Key cytokines and chemokines are instrumental in the progression of keratitis and have been the focus of significant research.[47]
At the outset of HSV-1 infection in the cornea, type I interferons (IFN-α and IFN-β) are among the earliest cytokines released by various cell types, including corneal epithelial cells. These interferons are crucial for the initial containment of viral spread, as their engagement with cell surface receptors activates downstream signaling pathways that enhance the expression of interferon-stimulated genes with antiviral capabilities. This process restricts the virus's ability to proliferate and fortifies neighboring cells against subsequent infection. The research underscores the necessity of type I interferons for effective control of HSV-1 replication and for mobilizing immune cells to the infection site.[48]
Interferon-γ (IFN-γ), or type II interferon, distinct in its production solely by immune system cells like Th1 cells, interacts with a different receptor than type I interferons. IFN-γ is instrumental in bolstering the innate immune response, augmenting cytokine production, enhancing phagocytosis, and upregulating class II MHC molecule expression. In the context of HSV-1 infection, IFN-γ works alongside IL-2 to provoke inflammation, facilitating the recruitment and priming of neutrophils and antigen-presenting cells.[49] Other cytokines (eg, IL-1, IL-6, and TNF-α) are recognized as primary instigators of inflammation within the cornea. Approaches to neutralizing these cytokines have shown promise in mitigating the severity of herpes stromal keratitis in animal models. IL-6, in particular, promotes corneal neovascularization by inducing vascular endothelial growth factor (VEGF) production. IL-17, produced by Th17 cells, similarly influences VEGF secretion, fostering angiogenesis postinfection.[50]
Chemokines like CXCL1 and CXCL2 specifically target neutrophils via the CXCR2 receptor, orchestrating their migration towards the cornea. Conversely, CXCL10, constitutively expressed in the cornea, operates through the CXCR3 receptor found on macrophages, dendritic cells, and activated T cells, playing a critical role in controlling viral spread and reducing herpes stromal keratitis severity. This chemokine is also vital in recruiting CD8+ T cells to the site of infection, offering protection against recurrence in experimental models.[51]
Amidst the array of proinflammatory cytokines, IL-10 emerges as a counter-regulatory force produced by corneal resident cells and regulatory T cells (Tregs) to mitigate immune-mediated damage. The administration of IL-1 postinfection has been shown to reduce the levels of inflammatory cytokines, diminish neutrophil infiltration, and decrease corneal opacity, highlighting its potential as a therapeutic agent in managing herpes stromal keratitis.[52]
Heparanase
The enzyme heparanase (HPSE), produced by host cells, plays a pivotal role in enhancing HSV infection and its associated pathogenic processes through various mechanisms. HPSE is crucial for regulating the balance of the extracellular matrix by facilitating the turnover of heparan sulfate, a cell-associated polysaccharide. This enzyme's expression is transcriptionally upregulated in the later stages of a productive HSV infection, a process partly reliant on the viral protein ICP34.5.[53] HPSE facilitates viral dissemination upon elevated expression by cleaving heparan sulfate moieties on the cell surface, releasing newly formed viral particles into the extracellular space. Given that heparan sulfate acts as a primary viral attachment receptor, its removal is essential for the virus's progeny to invade adjacent cells effectively. Although initially identified in the context of HSV-1, subsequent research has revealed HPSE's role in augmenting the release and pathogenicity of other viral agents, including dengue virus, human papillomavirus, respiratory syncytial virus, and porcine respiratory and reproductive syncytial virus. Strategies targeting HPSE inhibition, including applying the small molecule OGT 2115 or shRNA-mediated knockdown, have demonstrated a pronounced impact on reducing viral propagation and spread within the corneal tissue. Beyond its function in the viral spread, HPSE contributes to tissue damage and corneal inflammation by degrading the extracellular matrix and mobilizing growth factors like VEGF, which are typically bound to heparan sulfate chains.
This role of HPSE is corroborated by extensive research in oncology, highlighting its involvement in promoting angiogenesis, inflammation, and tumor metastasis.[54] Moreover, HPSE regulates proinflammatory cytokine transcription, including IL-1β, IL-6, and TNF-α, where its activation leads to elevated production of these cytokines. Viral infections further induce HPSE's nuclear translocation, suggesting its potential influence on the selective transcription of critical genes. While the precise mechanisms of HPSE's action are not fully understood, HPSE's multifaceted involvement in HSV infection and corneal inflammation positions it as a promising therapeutic target for managing the complex pathology of HSV infection.[50]
Mechanism of Viral Evasion
HSV-1 employs many strategies to elude host immune detection and manipulate cellular functions, facilitating its replication and maintenance within the host. A critical examination of the cellular pathways targeted by the virus reveals potential avenues for therapeutic intervention aimed at reinforcing these host defenses. The virus circumvents the host's early warning systems, namely the TLR, RIG-I-like receptor, and cytosolic DNA sensing pathways, which are primary alerts to viral presence. HSV-1 achieves this evasion by impairing the functionality of these sensing mechanisms or their associated signaling cascades, effectively remaining under the radar of host surveillance. A notable tactic involves the viral protein ICP0, which orchestrates the ubiquitination of crucial proteins within the host's defense network, such as the interferon-gamma inducible protein 16.[55] This modification essentially cloaks the virus from the innate immune system's detection. Furthermore, HSV-1 utilizes its viral kinases to modify the phosphorylation patterns of critical transcription factors, obstructing their ability to migrate to the nucleus and activate antiviral gene expression. This manipulation is exemplified in interferon regulatory factor 3 and nuclear factor-kB pathways alteration by the viral kinase US3. Beyond these mechanisms, HSV-1 disrupts a range of essential cellular defense processes, including the presentation of antigens via MHC class I, the DNA damage response, autophagy, the endoplasmic reticulum stress response, and the process of necroptosis. Each viral evasion tactic underscores the sophisticated interplay between HSV-1 and host cellular mechanisms, highlighting critical vulnerabilities within the host's immune defense. By advancing our understanding of these interactions, a promising potential exists to develop therapeutic strategies that enhance the host's cellular responses to combat HSV-1 infection and its persistence.[56]
Impact on the Ocular Surface and Cornea
A significant impact of HSV corneal infection is the degradation of corneal nerves, leading to a reduced sub-basal nerve plexus and, consequently, diminished corneal sensation. This sensory loss disrupts the normal blink reflex, potentially resulting in neurotrophic keratopathy, a condition characterized by epithelial damage, increased risk of superinfection, and the possibility of corneal dissolution. Studies utilizing in vivo confocal microscopy have identified a marked reduction in sub-basal nerve plexus density in individuals with HSV keratitis compared to healthy individuals, a change that aligns with decreases in corneal sensation; interestingly, even the nonaffected eyes of those with HSV keratitis show a reduction in nerve density relative to healthy controls.[57] This decrease in corneal nerve density begins early in the infection and progresses with the disease's duration. Experimental models have shown that mice experience a loss of corneal sensation as soon as 8 days postinfection, accompanied by an increase in the expression of semaphorin 7A, a nerve guidance molecule. However, reinnervation does not restore corneal sensation functionally. The mechanisms underlying HSV-induced damage to corneal nerves are subject to ongoing debate. In a research study using mice lacking the leukocyte marker P-selectin glycoprotein ligand-1, a more pronounced loss of corneal nerves was observed with fewer corneal leukocytes compared to their wild-type counterparts, suggesting viral activity rather than immune cell infiltration contributes to nerve damage.[58] Conversely, research by Yun et al found that mice deficient in CD4+ T cells exhibited nerve regeneration post-HSV infection, alongside restoration of the blink reflex and reduced inflammation. Additionally, studies have identified an overgrowth of sympathetic nerves following the loss of sensory nerves in HSV models; intervention to remove sympathetic nerve innervation significantly mitigated the severity of herpetic stromal keratitis.
Moreover, individuals with a history of unilateral HSV keratitis often experience bilateral tear dysfunction, even when the disease is dormant. Investigations have shown tear hyperosmolarity and decreased tear production and stability in the unaffected eyes of those with previous HSV keratitis, likely stemming from the impaired corneal sensation, which affects tear secretion. Consequently, those with unilateral HSV keratitis can suffer from ocular discomfort related to the onset of bilateral dry eye syndrome. Comparative studies have reported that individuals with unilateral HSV keratitis experience more significant discomfort and dry eye-related visual symptoms in their unaffected eyes than healthy controls. Such dry eye conditions or neuropathic pain are adverse effects of HSV's disruption of corneal nerves; distressingly, ocular discomfort can persist long after the resolution of active viral infection. Patients with dormant HSV keratitis have reported significant decreases in quality of life due to ocular pain, with those experiencing more frequent disease recurrences reporting enhanced pain-related quality of life degradation, possibly reflecting the memory of acute infection pain or the impact of chronic bilateral ocular surface disease from recurring keratitis.[59]
Histopathology
The histopathology of herpes simplex keratitis varies depending on the stage, acute or chronic, and type, epithelial, stromal, or endothelial of the infection. The following histopathological features are associated with each type of herpes simplex keratitis:
- Epithelial keratitis
- Dendritic Ulcers: Characteristic of early-stage herpes simplex keratitis, dendritic ulcers display swollen epithelial cells (ballooning degeneration) with ground-glass nuclei and chromatin marginalization. Multinucleated giant cells and Cowdry type A inclusion bodies may be observed.[60]
- Viral replication: Observable in the epithelial layer, leading to cell lysis and sloughing off, contributing to the formation of dendritic ulcers.[61]
- Necrotizing stromal keratitis: This form shows infiltration of inflammatory cells, including lymphocytes, plasma cells, and neutrophils, into the stroma. There may be stromal necrosis and edema.[26]
- Non-necrotizing (interstitial) stromal keratitis: This subtype of stromal keratitis is characterized by more diffuse stromal inflammation without significant necrosis. Fibrovascular tissue and neovascularization are joint, leading to corneal opacity.[62] Stromal disease is predominantly immune-mediated, with immune complexes and complement deposition in the cornea.[32]
- Endotheliitis
- Inflammatory cells: Within the aqueous humor, inflammatory cells can be seen as precipitates on the corneal endothelium.[63]
- Corneal edema: Corneal swelling and haziness may be present due to endothelial dysfunction and decreased endothelial cell density.[64]
- Neurotrophic keratopathy
- Corneal nerve loss: HSV infects the trigeminal ganglia, leading to decreased corneal sensation due to loss or damage to corneal nerves likely caused by a decrease in sub-basal nerve plexus density, which can be seen on histopathologic examination.[65]
Additional histologic examination findings include corneal neovascularization in the late stages of herpes simplex keratitis, corneal scarring, characterized by the proliferation of fibrous tissue within the cornea in chronic or recurrent infections, lipid deposits, and descemet's membrane folds and breaks due to severe endothelial dysfunction.[66][67][68] Understanding the histopathological features of herpes simplex keratitis is crucial for diagnosis, especially in atypical cases, and informs the choice of therapeutic strategies to prevent vision loss. Advanced imaging techniques, such as in vivo confocal microscopy, have complemented traditional histopathological examinations by allowing detailed observation of corneal changes in living patients, thus enhancing our understanding of herpes simplex keratitis pathology.[69]
History and Physical
Herpes simplex keratitis should be considered in patients complaining of acute, unilateral onset of eye pain, photophobia, visual blurring, and watery ocular discharge. History of prior episodes, including previous herpetic lesions in the orofacial or ocular area, should be obtained. Other essential clinical history includes contact lens use, prior corneal abrasions, recent stressors, including fever, ultraviolet laser treatment or ultraviolet light exposure, and topical or systemic corticosteroid use.[70]
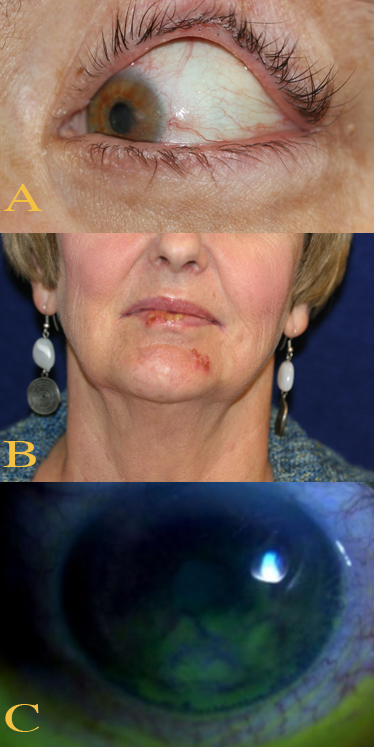
Primary herpes simplex keratitis infection is usually subclinical and may occasionally manifest as a mild, self-limiting blepharoconjunctivitis marked by inflammatory blisters or ulcers with lesions on the corneal epithelium.[71] Mild fever, malaise, or upper respiratory tract infection may also be present.[72] In recurrent herpes simplex keratitis, patients with dendritic or geographical keratitis complain of pain, foreign-body sensation, light sensitivity, redness, blurred vision, and corneal sensation gradually decreases.[73] Patients with disciform keratitis may experience similar symptoms; discomfort and redness are milder than in epithelial disease. Haloes are a common complaint at night in the presence of lights. Findings on physical examination differ according to the type of keratitis present, including epithelial keratitis, stromal disease, endothelins, and neurotrophic keratopathy; a slit lamp examination is required to identify and evaluate the lesions.[74] See Image. Herpes Simplex Keratitis Clinical Features.
Corneal Epithelial Disease
Cornel epithelial keratitis, dendritic or geographic, is characterized by active virus replication. Symptoms include mild to moderate discomfort, pain, redness, photophobia, watering, and blurred vision.[4] Cornel epithelial keratitis signs in chronological order include:
- Swollen opaque epithelial cells arranged in a coarse punctate or stellate pattern
- Central desquamation resulting in linear branching (dendritic) ulcers, frequently centrally located
- A central ulcer bed with ulcerated edges having characteristic terminal buds visible with special stains (eg, fluorescein and rose bengal)
- Rose bengal staining of virus-laden cells and the ulcer margin distinguishing herpetic ulceration from other conditions, particularly atypical recurrent corneal ulceration
- Progressively increasing ulceration resulting in a geographical or "amoeboid" configuration
- Mild subepithelial haze and scarring that may develop after healing
- Elevated intraocular pressure is not uncommon (Tonometry on the unaffected eye should be performed first using a disposable prism.)
- Reduced visual acuity
- Reduced corneal sensations
- Progressively enlarged ulcers and geographical or amoeboid configuration due to topical steroid use
- Mild anterior chamber inflammation
- Follicular conjunctivitis (may be caused by topical antivirals)
- Eyelid vesicular lesions
- Persistent punctate epithelial erosions with irregular epithelium that settles spontaneously a whorled epithelial appearance (can result after the defect heals, especially after prolonged topical antiviral use, and should not be mistaken for persistent active infection.)
- Recurrence in a corneal graft performed for a stromal scar [75][76]
Corneal Stromal and Endothelial Disease
The pathology of corneal stromal keratitis results from active HSV infection of keratocytes or endothelium or a hypersensitivity reaction to viral antigens in the cornea. Clinical features include:
- Occasionally eccentric lesion
- Central zone of stromal edema with overlying epithelial edema underlined by large granulomatous keratic precipitate
- Descemet membrane folds in severe cases
- In some cases, subepithelial and stromal scarring after healing
- Wessely immune ring of deep stromal infiltration indicating viral antigen deposition and host antibody complexes
- Elevated intraocular pressure (IOP)
- Blurred vision due to halos around the light
- Reduced corneal sensations, unlike other HSV infection types
- Discomfort and redness, though less severe than epithelial disease
- Faint stromal and subepithelial opacification and thinning ring in healed lesions
- Subepithelial and stromal scar worsening, resulting in superficial or deep vascularization in recurrent disciform keratitis [77]
Additionally, a history of epithelial dendrite or a geographical is not always present. Therefore, a differential diagnosis of acanthamoeba and fungal keratitis should always be considered. Mid-stromal scarring resulting from disciform keratitis is a significant cause of interstitial keratitis.[77]
Neurotrophic Keratopathy
A nonhealing epithelial defect, sometimes after prolonged topical treatment, may be associated with stromal melting and perforation due to failure of re-epithelization resulting from corneal anesthesia, which is often aggravated by drug toxicity.[78] Clinical features associated with neurotrophic keratopathy include a nonhealing epithelial defect, an early sign occurring after prolonged topical treatment, stromal opacification causing a grey opaque color, and overlying secondary bacterial or fungal infection.[78]
Necrotizing Stromal Keratitis
Necrotizing stromal keratitis is a rare condition that results from active viral replication within the stromal tissue due to immune-mediated inflammation, which plays a vital role in the pathology. Clinicians should differentiate this condition from the severe form of disciform keratitis. A spectrum of disease is associated with necrotizing stromal keratitis, including the overlap with neurotrophic keratopathy. Necrotizing stromal keratitis may also be caused by other infections.[15] Clinical features include stroma necrosis with profound interstitial opacification, acute anterior uveitis, keratitis precipitates below the area of active stromal infiltrate, underlying epithelial defects, progressive scarring, vascularization, and lipid deposition. Vesicles on the eyelids and skin, conjunctivitis, uveitis, and retinitis may also be seen on examination. [79]
Iridocyclitis
Herpetic iridocyclitis can result without signs of active corneal inflammation and may be associated with direct viral replication. The rise in intraocular pressure is common and often thought to result from trabeculitis. There can also be IOP elevation second to steroid use. The etiology can be missed in these cases unless an underlying history of herpes simplex keratitis is present. Patchy iris atrophy may provide a clue, and transillumination may reveal subtle lesions. The PCR from aqueous sampling is diagnostic. The treatment is primarily with topical steroids, but oral acyclovir may also be needed.[80]
Evaluation
Evaluating herpes simplex keratitis involves a combination of clinical assessment and laboratory tests to confirm the diagnosis, identify the type of herpes simplex virus (HSV) involved, and determine the extent of the infection. Herpes simplex keratitis is primarily diagnosed based on clinical features, but laboratory tests can provide definitive evidence of HSV infection.[37]
Laboratory Studies
Diagnostic laboratory studies are crucial for confirming the clinical suspicion of an HSV cornea infection. Several of the following techniques may be utilized to directly detect the virus's presence or its components in ocular specimens.[81]
Polymerase chain reaction
PCR is the preferred method for the laboratory diagnosis of herpes simplex keratitis due to its high sensitivity and specificity in detecting HSV DNA in ocular specimens (eg, corneal scrapings, tear fluid, or aqueous humor). The advantage of PCR is its ability to differentiate between HSV-1 and HSV-2. This testing modality is considered the gold standard for diagnosing viral infections of the eye, including herpes simplex keratitis. While other methods like viral culture and direct fluorescent antibody testing offer valuable information, their use is more limited than PCR.[82]
Viral culture
Viral culture involves inoculating ocular samples onto cell cultures susceptible to HSV. Observation of cytopathic effects consistent with herpesvirus infection confirms the presence of the virus. Allowing for viral isolation and antiviral susceptibility testing are some of the benefits of this test. However, viral culture is less sensitive than PCR and has a longer turnaround time, making it less useful for acute diagnosis.[83]
Direct fluorescent antibody testing
Direct fluorescent antibody testing uses fluorescently labeled antibodies that bind to HSV antigens in ocular samples. The advantages are that direct fluorescent antibody testing provides rapid results and is more specific than viral culture. This method is less sensitive than PCR and requires viable cells; therefore, sample handling is critical.[84]
Immunohistochemistry
Immunohistochemistry involves staining ocular tissue sections with antibodies against HSV antigens visualized under a microscope. This method is useful for detecting viral antigens in biopsy samples but is typically used for research or in cases where other diagnostic techniques are inconclusive.[85]
Serology
Serological tests may be used to detect serum HSV IgG and IgM to identify past or recent infections for epidemiological purposes or in a comprehensive assessment of the patient's immune status regarding HSV. However, serology is not typically used to diagnose active herpes simplex keratitis, as this test cannot differentiate between past exposure and active ocular infection. HSV antibodies are prevalent in the general population due to widespread HSV exposure.[86][84]
Sample collection and handling
Proper collection and handling of samples are paramount for accurate diagnosis. An experienced ophthalmologist should collect corneal scrapings to obtain sufficient material for analysis without causing additional injury to the eye. PCR and direct fluorescent antibody testing samples should be transported in viral transport media and processed as soon as possible to maintain viral integrity.[87]
Tear analysis
Analysis of tears for the presence of HSV DNA through PCR is a diagnostic approach that, while not routinely utilized, can be particularly revealing in specific instances. Tear analysis involves collecting tear fluid from patients suspected of having HSV infection and analyzing it for viral DNA. This technique's advantage lies in its noninvasiveness and the potential for early virus detection, especially in cases where corneal scraping is contraindicated or the specimen is insufficient. However, the sensitivity of tear analysis might be lower than that of direct corneal samples due to the tears' dilute nature and the virus's intermittent shedding.[82]
Anterior chamber paracentesis
This procedure involves sampling aqueous humor from the eye's anterior chamber for PCR testing, primarily in cases where HSV endotheliitis is suspected or when the clinical diagnosis remains uncertain. Anterior chamber paracentesis is a more invasive procedure requiring careful execution by an experienced ophthalmologist to avoid complications. The analysis of aqueous humor can provide definitive evidence of HSV infection within the eye's interior structures, which is crucial for diagnosing and managing cases with deep corneal or intraocular involvement where surface sampling may not indicate the underlying pathology.[88]
Additional Diagnostic Studies
Other evaluations that can be considered to assist in the diagnosis of herpes simplex keratitis, enabling targeted therapeutic interventions and monitoring of disease progression, include:
- In vivo confocal microscopy: In vivo confocal microscopy represents a ground-breaking, noninvasive imaging modality that offers high-resolution, real-time visualization of the corneal layers. This technique is instrumental in identifying microstructural changes in the cornea, including nerve fiber alterations, dendritic cell activation, and inflammatory infiltrates, indicative of herpes simplex keratitis. In vivo confocal microscopy's capacity to detect corneal nerve abnormalities aids in diagnosing neurotrophic keratitis, a complication of HSV infection characterized by reduced corneal sensation and poor healing. Furthermore, confocal microscopy can help differentiate herpes simplex keratitis from other forms of keratitis based on the unique cellular patterns observed, making this study a valuable tool in the comprehensive evaluation of corneal diseases.[89]
- Corneal sensitivity testing: Reduced corneal sensitivity is a hallmark of neurotrophic keratitis, often resulting from HSV infection's impact on corneal nerves. Corneal sensitivity testing, primarily using the Cochet-Bonnet esthesiometer, is a diagnostic procedure that quantitatively assesses the cornea's ability to perceive tactile stimuli. A decrease in corneal sensitivity supports the diagnosis of neurotrophic keratitis and can provide insight into the extent of nerve damage. This test is simple, noninvasive, and can be performed in the clinical setting, offering valuable information on the functional status of the corneal nerves. Monitoring changes in corneal sensitivity over time can also guide the effectiveness of treatment strategies to promote nerve regeneration and corneal healing.[90]
Treatment / Management
The condition present directs treatment. Herpes simplex keratitis treatment predominantly involves nucleoside analogs, typically purine or pyrimidine, that disrupt viral DNA. Most dendritic ulcers heal spontaneously without treatment, though significant scarring and vascularization may result.
Epithelial Keratitis Treatment
Topical therapy with acyclovir 3% ointment and ganciclovir 0.15% gel, each administered 5 times daily, is typically used initially for epithelial keratitis. Trifluridine can also be used up to 9 times a day. Approximately 99% of cases resolve within 2 weeks with topical treatment. Trifluridine can be reduced to 5 times a day after 1 week once the epithelium starts healing.[91][92] The drugs are nontoxic when given for up to 60 days. They have equivalent effects, preferably on virus-laden epithelial cells and penetrating effectively into the stroma, with approximately 99% of ulcers healing within 2 weeks duration. Idoxuridine and vidarabine are other older drugs that are less effective and can be more toxic.[93] Topical treatment toxicity is indicated by punctate epithelial erosions, wave-like whorled epitheliopathy, follicular conjunctivitis, and rarely punctual occlusion. Absent whirling in a persistent epithelial lesion stems from noncompliance with treatment.[94] Debridement may be required in resistant cases. With this procedure, the corneal surface is anesthetized and then wiped with a sterile cellulose sponge 2 mm beyond the edge of the ulcer to achieve virus-free margins. Debridement removes the virus-laden cells, protects the nearby healthy epithelium from infection, and removes the antigenic stimulus to stromal inflammation. This technique can be employed for dendritic but not geographical ulcers. A topical antiviral should be used in conjunction with the debridement.[95]
Immunodeficient patients, or patients who respond poorly to topical therapy, may benefit from taking oral antivirals. Acyclovir 200 to 400 mg 5 times daily for 5 to 10 days is recommended; additional antivirals include famciclovir and valacyclovir. Oral antiviral drugs are an alternative and effective treatment to topical drugs or for resistant cases. The newer oral antivirals are better tolerated than acyclovir and require less frequent dosing; the optimal regimen is not yet defined.[96] Interferon therapy is ineffective, but combining nucleoside antivirals with interferon therapy speeds healing.[97] Delayed healing and frequent recurrences indicate the presence of resistant viral strain, and alternative topical drug therapy and debridement may be required. A combined regimen of 2 topical agents with oral valaciclovir or famciclovir may be effective in refractory cases. A significant minority of cases are due to the varicella-zoster virus.[79] Topical steroids should be avoided in epithelial herpes keratitis, which can result in perforation.
Miotics and prostaglandin derivatives should be avoided as they promote viral activity and inflammation in general. Antiglaucoma drugs are usually required to control secondary glaucoma due to trabeculitis. Additionally, topical steroids should be avoided and should only be considered if significant disciform keratitis is present. Additional pharmacotherapy may be considered to manage symptoms and complications associated with epithelial keratitis. Cycloplegic medications (eg, topical homatropine 1% once or twice daily) can relieve pain and ciliary spasms in uveitis cases. Some clinicians recommend topical antibiotics to prevent secondary bacterial infection.
Disciform Keratitis Treatment
Disciform keratitis treatment regimens should be tailored individually based on the severity of inflammation; careful monitoring is required in all cases to reduce scarring progression. The patient should be told to take treatment immediately if there are signs of inflammation. Some clinicians believe minimal inflammation may not require urgent treatment and can be managed with cycloplegics alone.[98]
Initial treatment comprises topical steroids and antivirals used 4 times daily. The frequency of both is reduced in parallel over the next 4 weeks as improvement occurs. Topical steroids (eg, prednisolone 1% or dexamethasone 0.1%) can be used. The minimum steroid strength and duration needed to control the inflammation should be used. IOP should be monitored to prevent steroid-induced glaucoma. Cycloplegics can be used to improve comfort if necessary.[99] Following initial treatment regimens, patients are typically switched to prednisolone 0.5% or other weaker steroids once daily. Antivirals should be stopped altogether at this point. Periodic attempts should be made to stop steroid treatment as well. Some patients may need weak steroids such as fluorometholone 0.1% or loteprednol 0.2% on alternate days for several months, with the steroid regimen slowly tapered down until discontinued altogether.[100]
Steroid treatment should be minimal when active epithelial disease is present. Treatment with antivirals should be intensified. Initially, the topical antiviral should be given 5 times daily with steroids 2 or 3 times daily, titrated based on the clinical assessment. Oral antiviral treatment may be helpful, but the efficacy of the oral treatment is not established yet. Oral steroids are used to reduce severe stromal inflammation as an adjunct therapy to reduce steroid-induced IOP elevation. The steroid also helps in reducing the replication of infectious viral keratitis. Topical cyclosporin 0.05% may help taper steroid treatment, particularly in the presence of epithelial disease, and prevent steroid-induced IOP elevation. Fine needle diathermy and laser techniques have also been reported to be beneficial in addressing corneal neovascularization and improving vision.[101]
Neurotrophic Keratitis Treatment
Neurotrophic keratitis treatment is the same regimen recommended for persistent epithelial defects. Steroid use should remain at a bare minimum to prevent stromal necrolysis.[78]
Necrotizing Stromal Keratitis Treatment
The treatment is broadly similar to disciform keratitis, but more aggressive antiviral therapy may be beneficial. Oral antiviral supplementation is necessary, starting from a higher dose. The aim of treatment is the restoration of epithelial integrity.[102]
Keratoplasty Therapy
Keratoplasty is the last resort when the disease causes irreversible corneal damage. However, the recurrence of the reject on disease threatens graft survival. Keratoplasty should ideally be attempted after 3 months of inactivity when the disease is under control with topical steroids and oral acyclovir prophylaxis.[103] Therapeutic keratoplasty may be required in cases with overlying nonresolving secondary bacterial ulceration. Tectonic keratoplasty should be performed in cases with corneal perforation. Optical keratoplasty should only be attempted after 3 months of inactivity, and a high risk of recurrence and graft failure remains.[104]
A trial of rigid contact lenses must be given before surgery is performed. Recurrence and rejection are common and constantly threaten graft survival. Topical antivirals during a rejection episode may reduce epithelial rejection reactivation, but drug toxicity may delay re-epithelization. Oral acyclovir 400 mg 2 times daily increases graft survival and should be given to patients in whom optical penetrating keratoplasty is attempted for herpetic eye disease. The prophylaxis should be continued in patients with severe atopic eye disease but with no history of ocular HSV involvement. The treatment duration and optimum drug dose have not been established yet. Immunohistochemistry can confirm the presence of herpes antigen in the excised tissue.[105]
Herpes Simplex Keratitis Prophylaxis
Prophylaxis should be considered in patients with frequent recurrences or bilateral disease. Acyclovir 400 mg twice daily is standard, but a higher dose can be tried if necessary. Oral valaciclovir or famciclovir are alternatives to acyclovir. Prophylaxis reduces the recurrence rate of epithelial and stromal disease by about 50%. Long-term oral acyclovir reduction is very well tolerated. Prophylaxis is needed in patients with frequent debilitating recurrences if the disease is bilateral or unilateral. The prophylaxis can be continued for many years if deemed necessary for systemic infections. The prophylactic effect disappears or decreases when the drug is reduced. Excretion occurs through the kidneys, so renal function has to be monitored during long-term treatment.[106]
Alternatives to acyclovir include oral valaciclovir 500 mg once daily and famciclovir, which are as effective as acyclovir, require less frequent dosing and may be better tolerated. Long-term oral prophylaxis is preferred over topical drug administration as epithelial toxicity can occur, leading to mild burning and persistent discomfort. Allergy and punctal stenosis are significant side effects that must be considered. A vaccination strategy is being studied for the treatment of ocular disease.[106]
Differential Diagnosis
Other conditions that should be considered when evaluating herpes simplex keratitis include:
- Herpes zoster keratitis
- Psuedodendrite (healing corneal abrasion)
- Recurrent corneal erosion
- Acanthamoeba keratitis
- Vaccinia keratitis
- Epithelial rejection in a corneal graft
- Tyrosinaemia type 2
- Epithelial effects or impressions of soft contact lens use
- Toxic keratopathy secondary to topical medications
- Preservative-induced keratopathy (eg, polyquaternium-1)
Prognosis
The prognosis of HSV keratitis is generally favorable with therapy. The majority of dendritic ulcers heal spontaneously without treatment. Mild to moderate disease recovers with just 2 weeks of topical therapy reinforced with debridement if needed. However, prolonged epithelial or disciform keratitis may lead to scarring and vascularization, and visual acuity may be lost.[4]
Complications
A variety of complications may occur after herpes simplex keratitis. Secondary infections are not uncommon, and the most common is bacterial keratitis. Other complications include:
- Secondary glaucoma secondary to either inflammation or chronic steroid use
- Complicated cataract, possibly due to inflammation or chronic steroid use
- Iris atrophy secondary to kerato-uveiti
- Corneal thinning
- Perforation
- Corneal scarring
- Hypopyon
- Spillover intermediate or posterior uveitis
- Panuveitis
- Macular edema
- Progressive outer retinal necrosis
Postoperative and Rehabilitation Care
Good postoperative and rehabilitative care is necessary for patients undergoing keratoplasty. The patients should be started on topical antibiotics, oral antivirals, and adjuvants in therapeutic and tectonic keratoplasty cases.[107] In cases with optical penetrating keratoplasty, topical steroids under oral antiviral cover should be started along with adjuvant drugs. Topical steroids can be given hourly or every 2 hours for the first 48 hours and then should be tapered down in frequency every 3 months in this regimen. Topical antibiotics can be given 6 times daily for 15 days and tapered based on the clinical response. The adjuvant drugs required are topical homatropine 2 times per day and topical timolol 2 times per day to prevent uveitis and secondary glaucoma, respectively. The patient should be followed on days 1, 5, 14, and 28, then monthly for 3 months and 2 months postoperatively. The counseling should be stressed, and the patient should be told to follow up regularly for graft management and survival. The signs of graft rejection should be explained to the patient, and the importance of regular instillation of drugs should be stressed.[99]
Consultations
Any patient with a history of recurrent pain, redness, and defective vision should have an eye exam, suspecting possible herpetic keratitis. The general ophthalmologist should be able to recognize these cases in a routine clinical outpatient department.[4] In case of any doubt, the patient should be referred to a cornea specialist with experience managing microbial keratitis. Patients developing raised IOP secondary to trabeculitis or steroid-induced glaucoma should be referred to a cornea glaucoma specialist. In cases of developing iridocyclitis with spillover in the intermediate or posterior segment, a retinal examination is mandated by a retina specialist. A senior cataract surgeon should operate on patients having complicated cataracts to give excellent outcomes.[108]
Deterrence and Patient Education
Herpes simplex viruses are ubiquitous. Patients should be educated to avoid close contact with others when they have recurrent HSV lesions in any part of their body. Education should focus on performing good hand hygiene and hygiene in general to help mitigate transmission of the virus to others. Hand-eye contact should be avoided as much as possible, such as scratching the eye with dirty hands. Regular handwashing should be encouraged. Infected individuals should be isolated to minimize transmission to household members and other contacts. Once a diagnosis of herpes simplex keratitis is established, patients should be educated to recognize recurrences promptly, understand that management delay may lead to poor outcomes, and seek medical care to ensure appropriate care and treatment. Preventive therapy may also be necessary to minimize recurrences.[109]
Pearls and Other Issues
The physician should always consider herpetic simplex keratitis in any patient with eye pain. A thorough ocular examination, including a slit-lamp examination, is necessary to evaluate for corneal disease. The consequences of missing the diagnosis of herpes simplex keratitis and not receiving the appropriate education and treatment can have poor outcomes for the patient. Classic dendritic lesions may not initially be visualized at the time of the initial symptoms; a follow-up examination may be needed. In general, the index of suspicion should be high, especially in patients with classic symptoms of keratitis or with a prior history of herpes simplex keratitis.[14]
Enhancing Healthcare Team Outcomes
Herpes simplex keratitis is a common condition often initially evaluated by primary care or emergency room clinicians. A referral to an ophthalmologist is recommended for clinical suspicion or a diagnosis of herpes simplex keratitis; prompt diagnosis and treatment are necessary to minimize complications and the duration of the disease. Coordination among healthcare professionals is paramount to improve patient outcomes. Ophthalmology and dermatology clinicians, pharmacists, and other interprofessional team members should work together to manage the disease and its complications, implementing necessary measures to prevent its recurrence. Appropriate antiviral and antimicrobial therapy should be prescribed. Clinicians, along with pharmacists, should verify correct dosing as well as any drug interactions. The patients should be educated about their disease, management, and recurrence. Patients should also be educated on when to seek medical care. Close monitoring is essential until the symptoms have subsided and visual acuity has normalized. Interprofessional communication is the key to managing herpes simplex keratitis appropriately. Herpes keratitis management will be optimized by this interprofessional, collaborative approach, leading to better patient outcomes.