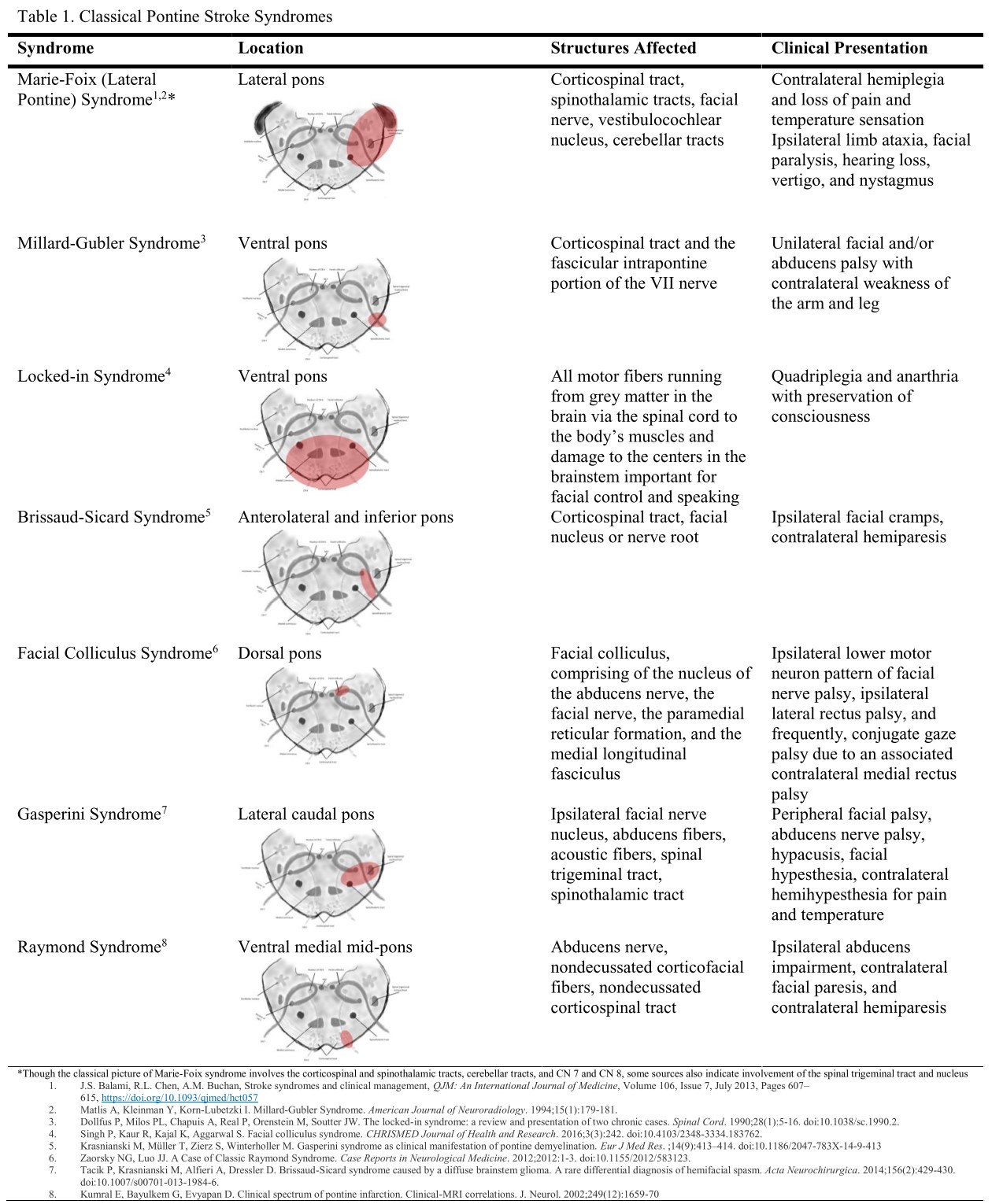
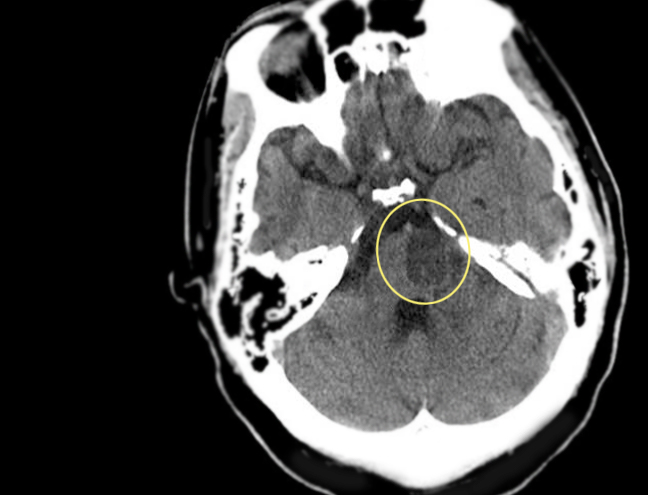
[1]
Kumral E, Bayülkem G, Evyapan D. Clinical spectrum of pontine infarction. Clinical-MRI correlations. Journal of neurology. 2002 Dec:249(12):1659-70
[PubMed PMID: 12529787]
[2]
Fisher CM,Caplan LR, Basilar artery branch occlusion: a cause of pontine infarction. Neurology. 1971 Sep;
[PubMed PMID: 5106254]
[4]
Caplan LR. Intracranial branch atheromatous disease: a neglected, understudied, and underused concept. Neurology. 1989 Sep:39(9):1246-50
[PubMed PMID: 2671793]
[5]
Gökçal E, Niftaliyev E, Baran G, Deniz Ç, Asil T. Progressive deficit in isolated pontine infarction: the association with etiological subtype, lesion topography and outcome. Acta neurologica Belgica. 2017 Sep:117(3):649-654. doi: 10.1007/s13760-017-0827-2. Epub 2017 Aug 3
[PubMed PMID: 28776182]
[6]
Kataoka S, Hori A, Shirakawa T, Hirose G. Paramedian pontine infarction. Neurological/topographical correlation. Stroke. 1997 Apr:28(4):809-15
[PubMed PMID: 9099201]
[7]
Kim JS, Lee JH, Im JH, Lee MC. Syndromes of pontine base infarction. A clinical-radiological correlation study. Stroke. 1995 Jun:26(6):950-5
[PubMed PMID: 7762044]
[8]
Lin Y, Zhang L, Bao J, Zhang B, Li H, Chen S, Sun S, Men X, Lu Z. Risk factors and etiological subtype analysis of brainstem infarctions. Journal of the neurological sciences. 2014 Mar 15:338(1-2):118-21. doi: 10.1016/j.jns.2013.12.028. Epub 2013 Dec 27
[PubMed PMID: 24411406]
[9]
Caplan LR. Lacunar infarction and small vessel disease: pathology and pathophysiology. Journal of stroke. 2015 Jan:17(1):2-6. doi: 10.5853/jos.2015.17.1.2. Epub 2015 Jan 30
[PubMed PMID: 25692102]
[10]
Baran G, Gultekin TO, Baran O, Deniz C, Katar S, Yildiz GB, Asil T. Association between etiology and lesion site in ischemic brainstem infarcts: a retrospective observational study. Neuropsychiatric disease and treatment. 2018:14():757-766. doi: 10.2147/NDT.S154224. Epub 2018 Mar 13
[PubMed PMID: 29559783]
Level 2 (mid-level) evidence
[11]
Barker-Collo S, Bennett DA, Krishnamurthi RV, Parmar P, Feigin VL, Naghavi M, Forouzanfar MH, Johnson CO, Nguyen G, Mensah GA, Vos T, Murray CJ, Roth GA, GBD 2013 Writing Group, GBD 2013 Stroke Panel Experts Group. Sex Differences in Stroke Incidence, Prevalence, Mortality and Disability-Adjusted Life Years: Results from the Global Burden of Disease Study 2013. Neuroepidemiology. 2015:45(3):203-14. doi: 10.1159/000441103. Epub 2015 Oct 28
[PubMed PMID: 26505984]
[12]
Lackland DT, Bachman DL, Carter TD, Barker DL, Timms S, Kohli H. The geographic variation in stroke incidence in two areas of the southeastern stroke belt: the Anderson and Pee Dee Stroke Study. Stroke. 1998 Oct:29(10):2061-8
[PubMed PMID: 9756582]
[13]
Pickle LW, Mungiole M, Gillum RF. Geographic variation in stroke mortality in blacks and whites in the United States. Stroke. 1997 Aug:28(8):1639-47
[PubMed PMID: 9259762]
[14]
Moon K, Albuquerque FC, Cole T, Gross BA, McDougall CG. Stroke prevention by endovascular treatment of carotid and vertebral artery dissections. Journal of neurointerventional surgery. 2017 Oct:9(10):952-957. doi: 10.1136/neurintsurg-2016-012565. Epub 2016 Sep 23
[PubMed PMID: 27663558]
[15]
Stojanov D, Vojinovic S, Aracki-Trenkic A, Tasic A, Benedeto-Stojanov D, Ljubisavljevic S, Vujnovic S. Imaging characteristics of cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL). Bosnian journal of basic medical sciences. 2015 Feb 9:15(1):1-8. doi: 10.17305/bjbms.2015.247. Epub 2015 Feb 9
[PubMed PMID: 25725137]
[16]
Janardhan V, Wolf PA, Kase CS, Massaro JM, D'Agostino RB, Franzblau C, Wilson PW. Anticardiolipin antibodies and risk of ischemic stroke and transient ischemic attack: the Framingham cohort and offspring study. Stroke. 2004 Mar:35(3):736-41
[PubMed PMID: 14764933]
[17]
Piechowski-Jóźwiak B, Bogousslavsky J. Posterior circulation strokes. Handbook of clinical neurology. 2009:93():537-58. doi: 10.1016/S0072-9752(08)93026-8. Epub
[PubMed PMID: 18804667]
[18]
Elyas AE, Bulters DO, Sparrow OC. Pathological laughter and crying in patients with pontine lesions. Journal of neurosurgery. Pediatrics. 2011 Dec:8(6):544-7. doi: 10.3171/2011.8.PEDS11265. Epub
[PubMed PMID: 22132910]
[19]
Saposnik G, Noel de Tilly L, Caplan LR. Pontine warning syndrome. Archives of neurology. 2008 Oct:65(10):1375-7. doi: 10.1001/archneur.65.10.1375. Epub
[PubMed PMID: 18852355]
[20]
Wintermark M, Fiebach J. Imaging of brain parenchyma in stroke. Handbook of clinical neurology. 2009:94():1011-9. doi: 10.1016/S0072-9752(08)94049-5. Epub
[PubMed PMID: 18793886]
[21]
Castellanos M, Castillo J, Dávalos A. Laboratory studies in the investigation of stroke. Handbook of clinical neurology. 2009:94():1081-95. doi: 10.1016/S0072-9752(08)94053-7. Epub
[PubMed PMID: 18793890]
[22]
Jickling GC, Sharp FR. Biomarker panels in ischemic stroke. Stroke. 2015 Mar:46(3):915-20. doi: 10.1161/STROKEAHA.114.005604. Epub 2015 Feb 5
[PubMed PMID: 25657186]
[23]
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019 Dec:50(12):e344-e418. doi: 10.1161/STR.0000000000000211. Epub 2019 Oct 30
[PubMed PMID: 31662037]
[24]
Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, McTaggart RA, Torbey MT, Kim-Tenser M, Leslie-Mazwi T, Sarraj A, Kasner SE, Ansari SA, Yeatts SD, Hamilton S, Mlynash M, Heit JJ, Zaharchuk G, Kim S, Carrozzella J, Palesch YY, Demchuk AM, Bammer R, Lavori PW, Broderick JP, Lansberg MG, DEFUSE 3 Investigators. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. The New England journal of medicine. 2018 Feb 22:378(8):708-718. doi: 10.1056/NEJMoa1713973. Epub 2018 Jan 24
[PubMed PMID: 29364767]
[25]
Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, Sila CA, Hassan AE, Millan M, Levy EI, Mitchell P, Chen M, English JD, Shah QA, Silver FL, Pereira VM, Mehta BP, Baxter BW, Abraham MG, Cardona P, Veznedaroglu E, Hellinger FR, Feng L, Kirmani JF, Lopes DK, Jankowitz BT, Frankel MR, Costalat V, Vora NA, Yoo AJ, Malik AM, Furlan AJ, Rubiera M, Aghaebrahim A, Olivot JM, Tekle WG, Shields R, Graves T, Lewis RJ, Smith WS, Liebeskind DS, Saver JL, Jovin TG, DAWN Trial Investigators. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. The New England journal of medicine. 2018 Jan 4:378(1):11-21. doi: 10.1056/NEJMoa1706442. Epub 2017 Nov 11
[PubMed PMID: 29129157]
[26]
Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D, ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. The New England journal of medicine. 2008 Sep 25:359(13):1317-29. doi: 10.1056/NEJMoa0804656. Epub
[PubMed PMID: 18815396]
[27]
National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. The New England journal of medicine. 1995 Dec 14:333(24):1581-7
[PubMed PMID: 7477192]
[28]
Erro ME, Gállego J, Herrera M, Bermejo B. Isolated pontine infarcts: etiopathogenic mechanisms. European journal of neurology. 2005 Dec:12(12):984-8
[PubMed PMID: 16324092]
[29]
Oh S, Bang OY, Chung CS, Lee KH, Chang WH, Kim GM. Topographic location of acute pontine infarction is associated with the development of progressive motor deficits. Stroke. 2012 Mar:43(3):708-13. doi: 10.1161/STROKEAHA.111.632307. Epub 2012 Feb 16
[PubMed PMID: 22343639]
[30]
Kunz S, Griese H, Busse O. Etiology and long-term prognosis of unilateral paramedian pontine infarction with progressive symptoms. European neurology. 2003:50(3):136-40
[PubMed PMID: 14530618]
[31]
Langhorne P, Stott DJ, Robertson L, MacDonald J, Jones L, McAlpine C, Dick F, Taylor GS, Murray G. Medical complications after stroke: a multicenter study. Stroke. 2000 Jun:31(6):1223-9
[PubMed PMID: 10835436]
Level 2 (mid-level) evidence
[32]
Chua KS, Kong KH. Functional outcome in brain stem stroke patients after rehabilitation. Archives of physical medicine and rehabilitation. 1996 Feb:77(2):194-7
[PubMed PMID: 8607746]
[33]
Yvonne Chan YF, Nagurka R, Richardson LD, Zaets SB, Brimacombe MB, Levine SR. Effectiveness of stroke education in the emergency department waiting room. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2010 May:19(3):209-215. doi: 10.1016/j.jstrokecerebrovasdis.2009.04.009. Epub
[PubMed PMID: 20434048]
[34]
Chaudhry B, Wang J, Wu S, Maglione M, Mojica W, Roth E, Morton SC, Shekelle PG. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Annals of internal medicine. 2006 May 16:144(10):742-52
[PubMed PMID: 16702590]
Level 2 (mid-level) evidence
[35]
Powers BJ, Danus S, Grubber JM, Olsen MK, Oddone EZ, Bosworth HB. The effectiveness of personalized coronary heart disease and stroke risk communication. American heart journal. 2011 Apr:161(4):673-80. doi: 10.1016/j.ahj.2010.12.021. Epub
[PubMed PMID: 21473965]
[36]
Stroke Unit Trialists' Collaboration. Organised inpatient (stroke unit) care for stroke. The Cochrane database of systematic reviews. 2013 Sep 11:2013(9):CD000197. doi: 10.1002/14651858.CD000197.pub3. Epub 2013 Sep 11
[PubMed PMID: 24026639]
Level 1 (high-level) evidence
[37]
Gorelick AR, Gorelick PB, Sloan EP. Emergency department evaluation and management of stroke: acute assessment, stroke teams and care pathways. Neurologic clinics. 2008 Nov:26(4):923-42, viii. doi: 10.1016/j.ncl.2008.05.008. Epub
[PubMed PMID: 19026897]
[38]
Lyrer PA. Acute stroke units and teams. Handbook of clinical neurology. 2009:94():1195-203. doi: 10.1016/S0072-9752(08)94058-6. Epub
[PubMed PMID: 18793895]