[1]
MOKLER CM, VAN ARMAN CG. Pharmacology of a new antiarrhythmic agent, gamma-diisopropyl-amino-alpha-phenyl-alpha-(2-pyridyl)-butyramide (SC-7031). The Journal of pharmacology and experimental therapeutics. 1962 Apr:136():114-24
[PubMed PMID: 14475124]
[2]
January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW, American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Journal of the American College of Cardiology. 2014 Dec 2:64(21):e1-76. doi: 10.1016/j.jacc.2014.03.022. Epub 2014 Mar 28
[PubMed PMID: 24685669]
Level 1 (high-level) evidence
[3]
Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL, American College of Cardiology/American Heart Association Task Force on Practice Guidelines, European Society of Cardiology Committee for Practice Guidelines, European Heart Rhythm Association, Heart Rhythm Society. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006 Aug 15:114(7):e257-354
[PubMed PMID: 16908781]
Level 1 (high-level) evidence
[4]
Fuster V, Rydén LE, Asinger RW, Cannom DS, Crijns HJ, Frye RL, Halperin JL, Kay GN, Klein WW, Lévy S, McNamara RL, Prystowsky EN, Wann LS, Wyse DG, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Russell RO, Smith SC Jr, Klein WW, Alonso-Garcia A, Blomström-Lundqvist C, de Backer G, Flather M, Hradec J, Oto A, Parkhomenko A, Silber S, Torbicki A, American College of Cardiology/American Heart Association Task Force on Practice Guidelines, European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation), North American Society of Pacing and Electrophysiology. ACC/AHA/ESC Guidelines for the Management of Patients With Atrial Fibrillation: Executive Summary A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation) Developed in Collaboration With the North American Society of Pacing and Electrophysiology. Circulation. 2001 Oct 23:104(17):2118-50
[PubMed PMID: 11673357]
Level 1 (high-level) evidence
[5]
Sherrid MV, Barac I, McKenna WJ, Elliott PM, Dickie S, Chojnowska L, Casey S, Maron BJ. Multicenter study of the efficacy and safety of disopyramide in obstructive hypertrophic cardiomyopathy. Journal of the American College of Cardiology. 2005 Apr 19:45(8):1251-8
[PubMed PMID: 15837258]
Level 2 (mid-level) evidence
[6]
Dan GA, Martinez-Rubio A, Agewall S, Boriani G, Borggrefe M, Gaita F, van Gelder I, Gorenek B, Kaski JC, Kjeldsen K, Lip GYH, Merkely B, Okumura K, Piccini JP, Potpara T, Poulsen BK, Saba M, Savelieva I, Tamargo JL, Wolpert C, ESC Scientific Document Group. Antiarrhythmic drugs-clinical use and clinical decision making: a consensus document from the European Heart Rhythm Association (EHRA) and European Society of Cardiology (ESC) Working Group on Cardiovascular Pharmacology, endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS) and International Society of Cardiovascular Pharmacotherapy (ISCP). Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2018 May 1:20(5):731-732an. doi: 10.1093/europace/eux373. Epub
[PubMed PMID: 29438514]
Level 3 (low-level) evidence
[7]
Karlson BW, Torstensson I, Abjörn C, Jansson SO, Peterson LE. Disopyramide in the maintenance of sinus rhythm after electroconversion of atrial fibrillation. A placebo-controlled one-year follow-up study. European heart journal. 1988 Mar:9(3):284-90
[PubMed PMID: 3289932]
[8]
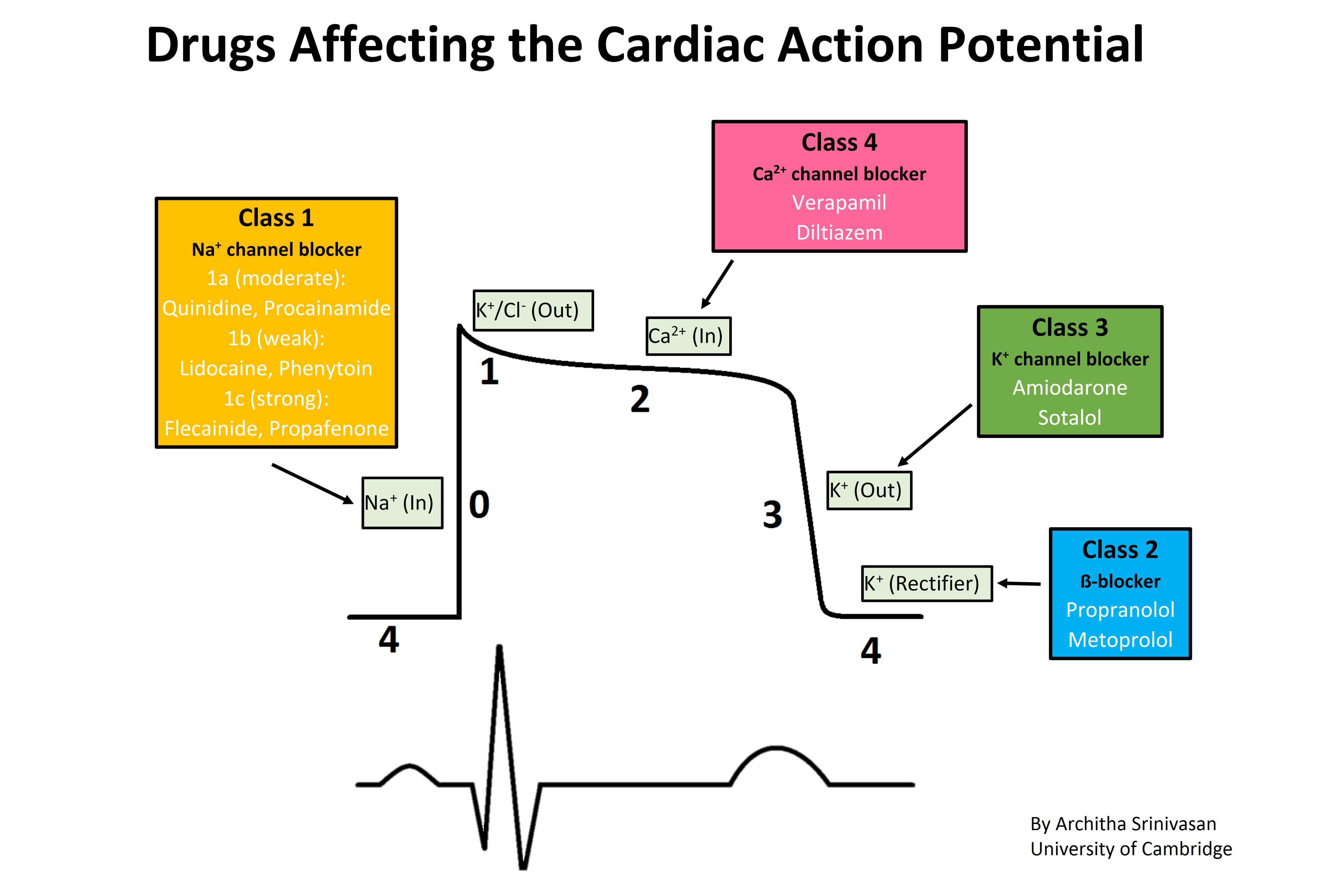
Vaughan Williams EM. A classification of antiarrhythmic actions reassessed after a decade of new drugs. Journal of clinical pharmacology. 1984 Apr:24(4):129-47
[PubMed PMID: 6144698]
[9]
Kus T, Sasyniuk BI. Electrophysiological actions of disopyramide phosphate on canine ventricular muscle and purkinje fibers. Circulation research. 1975 Dec:37(6):844-54
[PubMed PMID: 1192576]
[10]
. Oral disopyramide after admission to hospital with suspected acute myocardial infarction. U. K. Rythmodan Multicentre Study Group. Postgraduate medical journal. 1984 Feb:60(700):98-107
[PubMed PMID: 6369290]
[11]
Nicholls DP, Haybyrne T, Barnes PC. Intravenous and oral disopyramide after myocardial infarction. Lancet (London, England). 1980 Nov 1:2(8201):936-8
[PubMed PMID: 6107587]
[12]
Lafuente-Lafuente C, Valembois L, Bergmann JF, Belmin J. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. The Cochrane database of systematic reviews. 2015 Mar 28:(3):CD005049. doi: 10.1002/14651858.CD005049.pub4. Epub 2015 Mar 28
[PubMed PMID: 25820938]
Level 1 (high-level) evidence
[13]
Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez-Madrid A, Nikolaou N, Norekvål TM, Spaulding C, Van Veldhuisen DJ, ESC Scientific Document Group. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). European heart journal. 2015 Nov 1:36(41):2793-2867. doi: 10.1093/eurheartj/ehv316. Epub 2015 Aug 29
[PubMed PMID: 26320108]
[14]
Adler A, Fourey D, Weissler-Snir A, Hindieh W, Chan RH, Gollob MH, Rakowski H. Safety of Outpatient Initiation of Disopyramide for Obstructive Hypertrophic Cardiomyopathy Patients. Journal of the American Heart Association. 2017 May 26:6(6):. doi: 10.1161/JAHA.116.005152. Epub 2017 May 26
[PubMed PMID: 28550094]
[15]
Sherrid MV, Arabadjian M. A primer of disopyramide treatment of obstructive hypertrophic cardiomyopathy. Progress in cardiovascular diseases. 2012 May-Jun:54(6):483-92. doi: 10.1016/j.pcad.2012.04.003. Epub
[PubMed PMID: 22687589]
[16]
Conrad ME, Cumbie WG, Thrasher DR, Carpenter JT. Agranulocytosis associated with disopyramide therapy. JAMA. 1978 Oct 20:240(17):1857-8
[PubMed PMID: 691193]
[17]
Kim SY, Benowitz NL. Poisoning due to class IA antiarrhythmic drugs. Quinidine, procainamide and disopyramide. Drug safety. 1990 Nov-Dec:5(6):393-420
[PubMed PMID: 2285495]
[18]
Edmonds ME, Hayler AM. A case of intra-hepatic cholestasis after disopyramide therapy. European journal of clinical pharmacology. 1980 Oct:18(3):285-6
[PubMed PMID: 7439249]
Level 3 (low-level) evidence
[19]
Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2016 Nov:18(11):1609-1678
[PubMed PMID: 27567465]
[20]
Whiting B, Elliott HL. Disopyramide in renal impairment. Lancet (London, England). 1977 Dec 24-31:2(8052-8053):1363
[PubMed PMID: 74774]
[21]
Bryson SM, Whiting B, Lawrence JR. Disopyramide serum and pharmacologic effect kinetics applied to the assessment of bioavailability. British journal of clinical pharmacology. 1978 Nov:6(5):409-19
[PubMed PMID: 728284]
[22]
Bonde J, Graudal NA, Pedersen LE, Balsløv S, Angelo HR, Svendsen TL, Kampmann JP. Kinetics of disopyramide in decreased hepatic function. European journal of clinical pharmacology. 1986:31(1):73-7
[PubMed PMID: 3780831]
[23]
Echizen H, Saima S, Umeda N, Ishizaki T. Protein binding of disopyramide in liver cirrhosis and in nephrotic syndrome. Clinical pharmacology and therapeutics. 1986 Sep:40(3):274-80
[PubMed PMID: 3742934]
[24]
Gosselin B, Mathieu D, Chopin C, Wattel F, Dupuis B, Haguenoer JM, Desprez M. Acute intoxication with diisopyramide: clinical and experimental study by hemoperfusion an Amberlite XAD 4 resin. Clinical toxicology. 1980 Oct:17(3):439-49
[PubMed PMID: 7449357]
[25]
Hayler AM, Holt DW, Volans GN. Fatal overdosage with disopyramide. Lancet (London, England). 1978 May 6:1(8071):968-9
[PubMed PMID: 76895]
[26]
Sathyavagiswaran L. Fatal disopyramide intoxication from suicidal/accidental overdose. Journal of forensic sciences. 1987 Nov:32(6):1813-8
[PubMed PMID: 3323413]
[27]
Hinderling PH, Garrett ER. Pharmacokinetics of the antiarrhythmic disopyramide in healthy humans. Journal of pharmacokinetics and biopharmaceutics. 1976 Jun:4(3):199-230
[PubMed PMID: 978389]