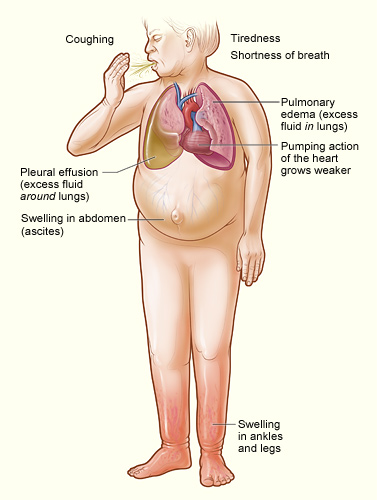
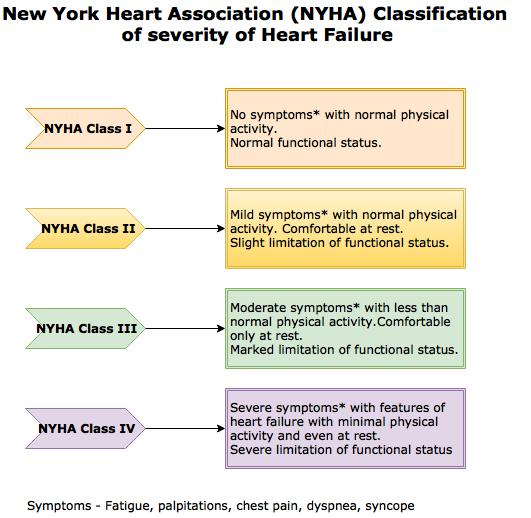
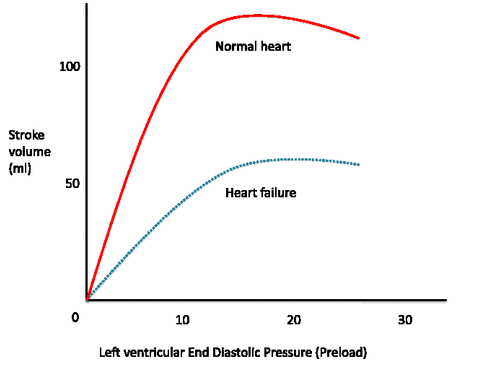
[1]
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European heart journal. 2016 Jul 14:37(27):2129-2200. doi: 10.1093/eurheartj/ehw128. Epub 2016 May 20
[PubMed PMID: 27206819]
[2]
WRITING COMMITTEE MEMBERS, Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013 Oct 15:128(16):e240-327. doi: 10.1161/CIR.0b013e31829e8776. Epub 2013 Jun 5
[PubMed PMID: 23741058]
Level 3 (low-level) evidence
[3]
Yusuf S, Rangarajan S, Teo K, Islam S, Li W, Liu L, Bo J, Lou Q, Lu F, Liu T, Yu L, Zhang S, Mony P, Swaminathan S, Mohan V, Gupta R, Kumar R, Vijayakumar K, Lear S, Anand S, Wielgosz A, Diaz R, Avezum A, Lopez-Jaramillo P, Lanas F, Yusoff K, Ismail N, Iqbal R, Rahman O, Rosengren A, Yusufali A, Kelishadi R, Kruger A, Puoane T, Szuba A, Chifamba J, Oguz A, McQueen M, McKee M, Dagenais G, PURE Investigators. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. The New England journal of medicine. 2014 Aug 28:371(9):818-27. doi: 10.1056/NEJMoa1311890. Epub
[PubMed PMID: 25162888]
[4]
Savarese G, Lund LH. Global Public Health Burden of Heart Failure. Cardiac failure review. 2017 Apr:3(1):7-11. doi: 10.15420/cfr.2016:25:2. Epub
[PubMed PMID: 28785469]
[5]
Purek L, Laule-Kilian K, Christ A, Klima T, Pfisterer ME, Perruchoud AP, Mueller C. Coronary artery disease and outcome in acute congestive heart failure. Heart (British Cardiac Society). 2006 May:92(5):598-602
[PubMed PMID: 16159982]
[6]
Phillips HR, O'Connor CM, Rogers J. Revascularization for heart failure. American heart journal. 2007 Apr:153(4 Suppl):65-73
[PubMed PMID: 17394905]
[7]
. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. The New England journal of medicine. 2017 Dec 21:377(25):2506. doi: 10.1056/NEJMx170008. Epub
[PubMed PMID: 29262284]
Level 1 (high-level) evidence
[8]
Writing Group Members, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, American Heart Association Statistics Committee, Stroke Statistics Subcommittee. Executive Summary: Heart Disease and Stroke Statistics--2016 Update: A Report From the American Heart Association. Circulation. 2016 Jan 26:133(4):447-54. doi: 10.1161/CIR.0000000000000366. Epub
[PubMed PMID: 26811276]
[9]
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017 Mar 7:135(10):e146-e603. doi: 10.1161/CIR.0000000000000485. Epub 2017 Jan 25
[PubMed PMID: 28122885]
[10]
Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, Jaarsma T, Krum H, Rastogi V, Rohde LE, Samal UC, Shimokawa H, Budi Siswanto B, Sliwa K, Filippatos G. Heart failure: preventing disease and death worldwide. ESC heart failure. 2014 Sep:1(1):4-25. doi: 10.1002/ehf2.12005. Epub
[PubMed PMID: 28834669]
[11]
Writing Group Members, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, American Heart Association Statistics Committee, Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016 Jan 26:133(4):e38-360. doi: 10.1161/CIR.0000000000000350. Epub 2015 Dec 16
[PubMed PMID: 26673558]
[12]
Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. Journal of the American College of Cardiology. 1993 Oct:22(4 Suppl A):6A-13A
[PubMed PMID: 8376698]
[13]
Huffman MD, Berry JD, Ning H, Dyer AR, Garside DB, Cai X, Daviglus ML, Lloyd-Jones DM. Lifetime risk for heart failure among white and black Americans: cardiovascular lifetime risk pooling project. Journal of the American College of Cardiology. 2013 Apr 9:61(14):1510-7. doi: 10.1016/j.jacc.2013.01.022. Epub
[PubMed PMID: 23500287]
[14]
Najafi F, Jamrozik K, Dobson AJ. Understanding the 'epidemic of heart failure': a systematic review of trends in determinants of heart failure. European journal of heart failure. 2009 May:11(5):472-9. doi: 10.1093/eurjhf/hfp029. Epub 2009 Feb 27
[PubMed PMID: 19251729]
Level 3 (low-level) evidence
[15]
Tsuji K, Sakata Y, Nochioka K, Miura M, Yamauchi T, Onose T, Abe R, Oikawa T, Kasahara S, Sato M, Shiroto T, Takahashi J, Miyata S, Shimokawa H, CHART-2 Investigators. Characterization of heart failure patients with mid-range left ventricular ejection fraction-a report from the CHART-2 Study. European journal of heart failure. 2017 Oct:19(10):1258-1269. doi: 10.1002/ejhf.807. Epub 2017 Mar 31
[PubMed PMID: 28370829]
[16]
Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. The New England journal of medicine. 2006 Jul 20:355(3):260-9
[PubMed PMID: 16855266]
[17]
Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA. 2006 Nov 8:296(18):2209-16
[PubMed PMID: 17090767]
[18]
Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. The New England journal of medicine. 2006 Jul 20:355(3):251-9
[PubMed PMID: 16855265]
[19]
Andersson C, Vasan RS. Epidemiology of heart failure with preserved ejection fraction. Heart failure clinics. 2014 Jul:10(3):377-88. doi: 10.1016/j.hfc.2014.04.003. Epub
[PubMed PMID: 24975902]
[20]
Tanai E, Frantz S. Pathophysiology of Heart Failure. Comprehensive Physiology. 2015 Dec 15:6(1):187-214. doi: 10.1002/cphy.c140055. Epub 2015 Dec 15
[PubMed PMID: 26756631]
[21]
Ponikowski P, van Veldhuisen DJ, Comin-Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD, CONFIRM-HF Investigators. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency†. European heart journal. 2015 Mar 14:36(11):657-68. doi: 10.1093/eurheartj/ehu385. Epub 2014 Aug 31
[PubMed PMID: 25176939]
[22]
Mant J, Doust J, Roalfe A, Barton P, Cowie MR, Glasziou P, Mant D, McManus RJ, Holder R, Deeks J, Fletcher K, Qume M, Sohanpal S, Sanders S, Hobbs FD. Systematic review and individual patient data meta-analysis of diagnosis of heart failure, with modelling of implications of different diagnostic strategies in primary care. Health technology assessment (Winchester, England). 2009 Jul:13(32):1-207, iii. doi: 10.3310/hta13320. Epub
[PubMed PMID: 19586584]
Level 1 (high-level) evidence
[23]
Kelder JC, Cramer MJ, van Wijngaarden J, van Tooren R, Mosterd A, Moons KG, Lammers JW, Cowie MR, Grobbee DE, Hoes AW. The diagnostic value of physical examination and additional testing in primary care patients with suspected heart failure. Circulation. 2011 Dec 20:124(25):2865-73. doi: 10.1161/CIRCULATIONAHA.111.019216. Epub 2011 Nov 21
[PubMed PMID: 22104551]
[24]
Gimelli A, Lancellotti P, Badano LP, Lombardi M, Gerber B, Plein S, Neglia D, Edvardsen T, Kitsiou A, Scholte AJ, Schröder S, Cosyns B, Gargiulo P, Zamorano JL, Perrone-Filardi P. Non-invasive cardiac imaging evaluation of patients with chronic systolic heart failure: a report from the European Association of Cardiovascular Imaging (EACVI). European heart journal. 2014 Dec 21:35(48):3417-25
[PubMed PMID: 25416326]
[25]
Agha SA, Kalogeropoulos AP, Shih J, Georgiopoulou VV, Giamouzis G, Anarado P, Mangalat D, Hussain I, Book W, Laskar S, Smith AL, Martin R, Butler J. Echocardiography and risk prediction in advanced heart failure: incremental value over clinical markers. Journal of cardiac failure. 2009 Sep:15(7):586-92. doi: 10.1016/j.cardfail.2009.03.002. Epub 2009 Apr 28
[PubMed PMID: 19700135]
[26]
Paterson I, Mielniczuk LM, O'Meara E, So A, White JA. Imaging heart failure: current and future applications. The Canadian journal of cardiology. 2013 Mar:29(3):317-28. doi: 10.1016/j.cjca.2013.01.006. Epub
[PubMed PMID: 23439018]
[27]
Butler J. The emerging role of multi-detector computed tomography in heart failure. Journal of cardiac failure. 2007 Apr:13(3):215-26
[PubMed PMID: 17448420]
[28]
Jimeno Sainz A, Gil V, Merino J, García M, Jordán A, Guerrero L. [Validity of Framingham criteria as a clinical test for systolic heart failure]. Revista clinica espanola. 2006 Nov:206(10):495-8
[PubMed PMID: 17203567]
[29]
Dassanayaka S, Jones SP. Recent Developments in Heart Failure. Circulation research. 2015 Sep 11:117(7):e58-63. doi: 10.1161/CIRCRESAHA.115.305765. Epub
[PubMed PMID: 26358111]
[30]
Roberts E, Ludman AJ, Dworzynski K, Al-Mohammad A, Cowie MR, McMurray JJ, Mant J, NICE Guideline Development Group for Acute Heart Failure. The diagnostic accuracy of the natriuretic peptides in heart failure: systematic review and diagnostic meta-analysis in the acute care setting. BMJ (Clinical research ed.). 2015 Mar 4:350():h910. doi: 10.1136/bmj.h910. Epub 2015 Mar 4
[PubMed PMID: 25740799]
Level 1 (high-level) evidence
[31]
SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. The New England journal of medicine. 2015 Nov 26:373(22):2103-16. doi: 10.1056/NEJMoa1511939. Epub 2015 Nov 9
[PubMed PMID: 26551272]
Level 1 (high-level) evidence
[32]
Pradhan A, Vohra S, Vishwakarma P, Sethi R. Review on sodium-glucose cotransporter 2 inhibitor (SGLT2i) in diabetes mellitus and heart failure. Journal of family medicine and primary care. 2019 Jun:8(6):1855-1862. doi: 10.4103/jfmpc.jfmpc_232_19. Epub
[PubMed PMID: 31334145]
[33]
Dini FL, Ghio S, Klersy C, Rossi A, Simioniuc A, Scelsi L, Genta FT, Cicoira M, Tavazzi L, Temporelli PL. Effects on survival of loop diuretic dosing in ambulatory patients with chronic heart failure using a propensity score analysis. International journal of clinical practice. 2013 Jul:67(7):656-64. doi: 10.1111/ijcp.12144. Epub
[PubMed PMID: 23758444]
[34]
CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The New England journal of medicine. 1987 Jun 4:316(23):1429-35
[PubMed PMID: 2883575]
Level 3 (low-level) evidence
[35]
SOLVD Investigators, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The New England journal of medicine. 1991 Aug 1:325(5):293-302
[PubMed PMID: 2057034]
[36]
McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. The New England journal of medicine. 2014 Sep 11:371(11):993-1004. doi: 10.1056/NEJMoa1409077. Epub 2014 Aug 30
[PubMed PMID: 25176015]
[37]
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members, Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European journal of heart failure. 2016 Aug:18(8):891-975. doi: 10.1002/ejhf.592. Epub 2016 May 20
[PubMed PMID: 27207191]
[38]
Wang Y, Zhou R, Lu C, Chen Q, Xu T, Li D. Effects of the Angiotensin-Receptor Neprilysin Inhibitor on Cardiac Reverse Remodeling: Meta-Analysis. Journal of the American Heart Association. 2019 Jul 2:8(13):e012272. doi: 10.1161/JAHA.119.012272. Epub 2019 Jun 26
[PubMed PMID: 31240976]
Level 1 (high-level) evidence
[39]
Chatterjee S, Biondi-Zoccai G, Abbate A, D'Ascenzo F, Castagno D, Van Tassell B, Mukherjee D, Lichstein E. Benefits of β blockers in patients with heart failure and reduced ejection fraction: network meta-analysis. BMJ (Clinical research ed.). 2013 Jan 16:346():f55. doi: 10.1136/bmj.f55. Epub 2013 Jan 16
[PubMed PMID: 23325883]
Level 1 (high-level) evidence
[40]
Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ, Parkhomenko A, Borbola J, Cohen-Solal A, Dumitrascu D, Ferrari R, Lechat P, Soler-Soler J, Tavazzi L, Spinarova L, Toman J, Böhm M, Anker SD, Thompson SG, Poole-Wilson PA, SENIORS Investigators. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). European heart journal. 2005 Feb:26(3):215-25
[PubMed PMID: 15642700]
Level 1 (high-level) evidence
[41]
Packer M, Fowler MB, Roecker EB, Coats AJ, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Staiger C, Holcslaw TL, Amann-Zalan I, DeMets DL, Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study Group. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002 Oct 22:106(17):2194-9
[PubMed PMID: 12390947]
Level 1 (high-level) evidence
[42]
Phelan D, Thavendiranathan P, Collier P, Marwick TH. Aldosterone antagonists improve ejection fraction and functional capacity independently of functional class: a meta-analysis of randomised controlled trials. Heart (British Cardiac Society). 2012 Dec:98(23):1693-700. doi: 10.1136/heartjnl-2012-302178. Epub 2012 Jul 12
[PubMed PMID: 22791658]
Level 1 (high-level) evidence
[43]
Rathore SS, Wang Y, Krumholz HM. Sex-based differences in the effect of digoxin for the treatment of heart failure. The New England journal of medicine. 2002 Oct 31:347(18):1403-11
[PubMed PMID: 12409542]
[44]
Borer JS, Böhm M, Ford I, Komajda M, Tavazzi L, Sendon JL, Alings M, Lopez-de-Sa E, Swedberg K, SHIFT Investigators. Effect of ivabradine on recurrent hospitalization for worsening heart failure in patients with chronic systolic heart failure: the SHIFT Study. European heart journal. 2012 Nov:33(22):2813-20. doi: 10.1093/eurheartj/ehs259. Epub 2012 Aug 27
[PubMed PMID: 22927555]
[45]
McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM, DAPA-HF Trial Committees and Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. The New England journal of medicine. 2019 Nov 21:381(21):1995-2008. doi: 10.1056/NEJMoa1911303. Epub 2019 Sep 19
[PubMed PMID: 31535829]
[46]
Rotunno R, Oppo I, Saetta G, Aveta P, Bruno S. NSAIDs and heart failure: A dangerous relationship. Monaldi archives for chest disease = Archivio Monaldi per le malattie del torace. 2018 Jun 7:88(2):950. doi: 10.4081/monaldi.2018.950. Epub 2018 Jun 7
[PubMed PMID: 29877658]
[47]
Girouard C, Grégoire JP, Poirier P, Moisan J. Effect of contraindicated drugs for heart failure on hospitalization among seniors with heart failure: A nested case-control study. Medicine. 2017 Mar:96(9):e6239. doi: 10.1097/MD.0000000000006239. Epub
[PubMed PMID: 28248890]
Level 2 (mid-level) evidence
[48]
Hernandez AV, Usmani A, Rajamanickam A, Moheet A. Thiazolidinediones and risk of heart failure in patients with or at high risk of type 2 diabetes mellitus: a meta-analysis and meta-regression analysis of placebo-controlled randomized clinical trials. American journal of cardiovascular drugs : drugs, devices, and other interventions. 2011:11(2):115-28. doi: 10.2165/11587580-000000000-00000. Epub
[PubMed PMID: 21294599]
Level 1 (high-level) evidence
[49]
Connolly SJ, Camm AJ, Halperin JL, Joyner C, Alings M, Amerena J, Atar D, Avezum Á, Blomström P, Borggrefe M, Budaj A, Chen SA, Ching CK, Commerford P, Dans A, Davy JM, Delacrétaz E, Di Pasquale G, Diaz R, Dorian P, Flaker G, Golitsyn S, Gonzalez-Hermosillo A, Granger CB, Heidbüchel H, Kautzner J, Kim JS, Lanas F, Lewis BS, Merino JL, Morillo C, Murin J, Narasimhan C, Paolasso E, Parkhomenko A, Peters NS, Sim KH, Stiles MK, Tanomsup S, Toivonen L, Tomcsányi J, Torp-Pedersen C, Tse HF, Vardas P, Vinereanu D, Xavier D, Zhu J, Zhu JR, Baret-Cormel L, Weinling E, Staiger C, Yusuf S, Chrolavicius S, Afzal R, Hohnloser SH, PALLAS Investigators. Dronedarone in high-risk permanent atrial fibrillation. The New England journal of medicine. 2011 Dec 15:365(24):2268-76. doi: 10.1056/NEJMoa1109867. Epub 2011 Nov 14
[PubMed PMID: 22082198]
[50]
Heart Failure Society of America, Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JG, Starling RC, Stevenson WG, Tang WH, Teerlink JR, Walsh MN. HFSA 2010 Comprehensive Heart Failure Practice Guideline. Journal of cardiac failure. 2010 Jun:16(6):e1-194. doi: 10.1016/j.cardfail.2010.04.004. Epub
[PubMed PMID: 20610207]
Level 1 (high-level) evidence
[51]
Lund LH, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI, Dobbels F, Goldfarb SB, Levvey BJ, Meiser B, Yusen RD, Stehlik J, International Society of Heart and Lung Transplantation. The registry of the International Society for Heart and Lung Transplantation: thirty-first official adult heart transplant report--2014; focus theme: retransplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2014 Oct:33(10):996-1008. doi: 10.1016/j.healun.2014.08.003. Epub 2014 Aug 14
[PubMed PMID: 25242124]
[52]
Lietz K, Long JW, Kfoury AG, Slaughter MS, Silver MA, Milano CA, Rogers JG, Naka Y, Mancini D, Miller LW. Outcomes of left ventricular assist device implantation as destination therapy in the post-REMATCH era: implications for patient selection. Circulation. 2007 Jul 31:116(5):497-505
[PubMed PMID: 17638928]
[53]
Jones RH, Velazquez EJ, Michler RE, Sopko G, Oh JK, O'Connor CM, Hill JA, Menicanti L, Sadowski Z, Desvigne-Nickens P, Rouleau JL, Lee KL, STICH Hypothesis 2 Investigators. Coronary bypass surgery with or without surgical ventricular reconstruction. The New England journal of medicine. 2009 Apr 23:360(17):1705-17. doi: 10.1056/NEJMoa0900559. Epub 2009 Mar 29
[PubMed PMID: 19329820]
[54]
Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM, TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. The New England journal of medicine. 2014 Apr 10:370(15):1383-92. doi: 10.1056/NEJMoa1313731. Epub
[PubMed PMID: 24716680]
[55]
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3:145(18):e895-e1032. doi: 10.1161/CIR.0000000000001063. Epub 2022 Apr 1
[PubMed PMID: 35363499]
Level 1 (high-level) evidence
[56]
Abraham WT, Fonarow GC, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB, OPTIMIZE-HF Investigators and Coordinators. Predictors of in-hospital mortality in patients hospitalized for heart failure: insights from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Journal of the American College of Cardiology. 2008 Jul 29:52(5):347-56. doi: 10.1016/j.jacc.2008.04.028. Epub
[PubMed PMID: 18652942]
[57]
Ouwerkerk W, Voors AA, Zwinderman AH. Factors influencing the predictive power of models for predicting mortality and/or heart failure hospitalization in patients with heart failure. JACC. Heart failure. 2014 Oct:2(5):429-36. doi: 10.1016/j.jchf.2014.04.006. Epub 2014 Sep 3
[PubMed PMID: 25194294]
[58]
Pocock SJ, Wang D, Pfeffer MA, Yusuf S, McMurray JJ, Swedberg KB, Ostergren J, Michelson EL, Pieper KS, Granger CB. Predictors of mortality and morbidity in patients with chronic heart failure. European heart journal. 2006 Jan:27(1):65-75
[PubMed PMID: 16219658]
[59]
Xiao YF. Cardiac arrhythmia and heart failure: From bench to bedside. Journal of geriatric cardiology : JGC. 2011 Sep:8(3):131-2. doi: 10.3724/SP.J.1263.2011.00131. Epub
[PubMed PMID: 22783298]
[60]
Haeusler KG, Laufs U, Endres M. Chronic heart failure and ischemic stroke. Stroke. 2011 Oct:42(10):2977-82. doi: 10.1161/STROKEAHA.111.628479. Epub 2011 Sep 8
[PubMed PMID: 21903953]
[61]
Divani AA, Vazquez G, Asadollahi M, Qureshi AI, Pullicino P. Nationwide frequency and association of heart failure on stroke outcomes in the United States. Journal of cardiac failure. 2009 Feb:15(1):11-6. doi: 10.1016/j.cardfail.2008.09.001. Epub 2008 Dec 25
[PubMed PMID: 19181288]
[62]
Kim W, Kim EJ. Heart Failure as a Risk Factor for Stroke. Journal of stroke. 2018 Jan:20(1):33-45. doi: 10.5853/jos.2017.02810. Epub 2018 Jan 31
[PubMed PMID: 29402070]
[63]
Beemath A, Stein PD, Skaf E, Al Sibae MR, Alesh I. Risk of venous thromboembolism in patients hospitalized with heart failure. The American journal of cardiology. 2006 Sep 15:98(6):793-5
[PubMed PMID: 16950187]
[64]
Sandek A, Swidsinski A, Schroedl W, Watson A, Valentova M, Herrmann R, Scherbakov N, Cramer L, Rauchhaus M, Grosse-Herrenthey A, Krueger M, von Haehling S, Doehner W, Anker SD, Bauditz J. Intestinal blood flow in patients with chronic heart failure: a link with bacterial growth, gastrointestinal symptoms, and cachexia. Journal of the American College of Cardiology. 2014 Sep 16:64(11):1092-102. doi: 10.1016/j.jacc.2014.06.1179. Epub
[PubMed PMID: 25212642]
[65]
Udani SM, Koyner JL. The effects of heart failure on renal function. Cardiology clinics. 2010 Aug:28(3):453-65. doi: 10.1016/j.ccl.2010.04.004. Epub
[PubMed PMID: 20621250]
[66]
DeWalt DA, Schillinger D, Ruo B, Bibbins-Domingo K, Baker DW, Holmes GM, Weinberger M, Macabasco-O'Connell A, Broucksou K, Hawk V, Grady KL, Erman B, Sueta CA, Chang PP, Cene CW, Wu JR, Jones CD, Pignone M. Multisite randomized trial of a single-session versus multisession literacy-sensitive self-care intervention for patients with heart failure. Circulation. 2012 Jun 12:125(23):2854-62. doi: 10.1161/CIRCULATIONAHA.111.081745. Epub 2012 May 9
[PubMed PMID: 22572916]
Level 1 (high-level) evidence