Continuing Education Activity
Retinopathy refers to pathological alterations to the retina resulting from a variety of causes, including environmental conditions and genetic factors. As the tissue with the highest oxygen consumption and the highest content of polyunsaturated fatty acid, the retina is especially susceptible damage from oxidative stress. In mammals, the retinal and choroidal circulatory systems provide oxygen and nutrients to the retina, and most major causes of retinopathy involve damage to these systems. This activity explains when retinopathy should be considered on a differential diagnosis and reviews the proper evaluation and management of this condition. This activity stresses the role of the interprofessional team in caring for patients with this condition.
Objectives:
- Explain the pathophysiologic basis of retinopathy.
- Review the complications of untreated retinopathy.
- Outline the treatment strategies for retinopathy.
- Describe how enhanced coordination of the interprofessional team can lead to more rapid detection of retinopathy and subsequently enhance the detection of pathology and allow for treatment when indicated.
Introduction
The retina contains photoreceptor cells that function in the process of visual transduction, i.e., transforming light signals to nerve impulses eventually transmitted from the optic nerve to the brain forming an image. There are two types of photoreceptor cells, rod, and cones. The cone cells are for color vision and the rod cells, which function well in low light conditions, i.e., scotopic vision. The macula is the small part of the retina responsible for central vision (see Figure), and it has a very high density of cone cells thereby providing high visual acuity. Retinopathy is not a specific disease but refers to pathological alterations to the retina resulting from a variety of causes, environmental conditions, and genetic factors. The retina is especially susceptible to the effects of oxidative stress damage since it has the highest content of polyunsaturated fatty acids of any tissue as well as the highest oxygen consumption.[1]
In mammals, the retina is supplied with oxygen and nutrients by the retinal and choroidal circulatory systems, and most major causes of retinopathy involve damage to these systems.[2] The retinal circulation supplies the inner (towards the center of the eye) half of the retina and the choroidal circulation supplies the outer half of the retina. Light must pass through the retinal capillaries (and some neuronal layers) before striking the photoreceptors (see Figure). The choroid lies behind the retinal pigment epithelium (RPE). The RPE, the macula, and anterior segment of the optic nerve depend on the choroidal circulation for oxygen and nutrients (see Figure). Lack of sufficient blood flow in the retinal circulation can lead to optic neuropathy and vision loss. Rupture of the retinal capillaries can lead to bleeding into the vitreous humor (vitreous hemorrhage) and vision loss.[3]
Etiology
Diabetic retinopathy (DR) is the commonest form of retinopathy as well as the most common cause of adult vision loss and blindness.[4] Poor glycemic control and hypertension contribute to DR. Diabetic retinopathy consists of two types, a proliferative form (PDR) and a nonproliferative form (NPDR). NPDR is caused by microaneurysms in the retinal circulation resulting in leakage of fluids and blood into the retina. When this leakage occurs in the macula, the result is macular edema with subsequent impairment of vision. PDR is the more advanced type of diabetic retinopathy and results from the proliferation (neovascularization) of leaky retinal vasculature. Ocular neovascularization is a major factor causing a shift from the non-proliferative form of diabetic retinopathy to the more pernicious proliferative form. The new and fragile vessels can cause a vitreous hemorrhage thereby preventing light from reaching the photoreceptors.
Hypertensive retinopathy is also very prevalent and important since its prime driving factor, hypertension is present in about one-third of USA adults and is a risk factor for cardiovascular disease.[5] Either chronic or acute episodes of hypertension can contribute to hypertensive retinopathy. Chronic hypertension causes a constriction of the retinal vasculature (i.e., an increase in vascular tone) which can eventually result in damage to vascular endothelial cells. Endothelial cell damage can, in turn, result in the final “sclerotic” phase of hypertensive retinopathy.
Retinopathy of prematurity (ROP) is a major worldwide cause of blindness and vision loss in premature, low birth weight and extremely low birth weight newborns. Although rare, ROP “like” signs can also occur is full-term newborns.[6] ROP is an “oxidative stress disease” of neonatology and has been etiologically linked to the necessity for some premature infants to receive supplemental oxygen therapy.[7][8]
Vascular endothelial growth factor (VEGF) plays a key role in many retinopathies. Hypoxia induces VEGF expression, and VEGF stimulates angiogenesis.[8] Oxygen therapy for ROP results in transient hyperoxia as well as an initial suppression of VEGF which, in turn, results in incomplete and abnormal retinal vasculature as well as endothelial cell death. Subsequent closure of the retinal vasculature results in hypoxia with an increased VEGF synthesis resulting in neovascularization.
Age-related macular degeneration (AMD) results in impairment of central (but not peripheral) vision and is caused by damage to the macula. There are two major variants of AMD, “wet” (or exudative) and “dry” (or atrophic) with “dry” accounting for about 80 to 90% of all AMD cases. Although accounting for only the remaining 10 to 20% of all AMD cases, wet AMD is responsible for about 90% of severe vision loss.[9] The intracellular accumulation of oxidized lipid pigments (lipofuscin) in the retinal pigment epithelium (RPE) is known to correlate with AMD.[10] The RPE is a monolayer of cells behind the photoreceptors (see Figure) and functions to phagocytize outer photoreceptor segments that are shed daily. The etiology of AMD is an active area of research. Lipofuscin accumulation resulting from an impairment of RPE phagocytosis has been thought to be important in AMD pathogenesis, and recent work supports this view.[11]
Similarly, the extracellular accumulation of drusen between the RPE and the Bruch’s membrane is also a risk factor for AMD. Drusen, like lipofuscin, is a complex chemical mixture containing oxidatively modified proteins. Some of the oxidatively modified proteins in drusen crosslink to oxidative products of docosahexaenoic (22:6n3) which is polyunsaturated fatty acid with a high content in rod outer segment phospholipids.[12]
Epidemiology
All of the retinopathies reviewed here are leading worldwide causes of vision loss. Hypertensive retinopathy, diabetic retinopathy, and AMD will be increasingly important due to aging populations with increased incidences of hypertension and diabetes. Advances in the treatment of prematurity have increased survival rates in ROP, thereby contributing to increased ROP incidence. Moreover, most retinopathies have a multifactorial etiologies with lifestyle, environmental factors, and genetics all playing important roles.
About one-third of individuals with diabetes have signs of diabetic retinopathy.[13] Both hyperglycemia and hypertension are established risks for diabetic retinopathy. Inflammation and oxidative stress may also be risk factors for diabetic retinopathy.[13]
Hypertensive retinopathy is present in about two-thirds of all hypertensive outpatients with hypertensive duration, age, and systolic blood pressure all being significant risks.[14] In contrast, gender and BMI (body mass index) are not significant risk factors for hypertensive retinopathy.
Age-Related macular degeneration (AMD) is a prime cause of blindness in the elderly (older than 60 years old) and is less prevalent in people with African ancestry. Early AMD is more prevalent in people with European ancestry compared to Asians.[15]
A very large-scale and comprehensive study (the USA and Canada) on the incidence of retinopathy of prematurity shows that at least 40% of at-risk-premature infants develop some stage of ROP but that most of these newborns improve without treatment.[16] About 8 to 10% do, however, develop severe ROP and these cases occur primarily in newborns with a birth weight under 1250 g.
Evaluation
The evaluation of most retinopathies relies on ophthalmoscopy (also called funduscopy) which can also evaluate the optic disc and the vitreous humor. Eye dilation is necessary for accurate assessment of any retinopathy. Ophthalmoscopy comes in few varieties, i.e., direct, indirect, slit-lamp and pantropic. A direct ophthalmoscope is a small handheld device with its own source of illumination and is typically used most in a primary care setting. In patients with mild hypertension, direct ophthalmoscopy has been found to have a significant inter- and intra-observer variability.[17]
An indirect ophthalmoscope is also a handheld device but has a separate source of illumination from a headband and is almost universally performed by an ophthalmologist. The indirect method provides a wider field of vision for the back of the eye than direct ophthalmoscopy. Pan ophthalmoscopy is a newer form of direct ophthalmoscopy that can be used without pupil dilation while providing a broad view of the fundus. Slit-lamp ophthalmoscopy utilizes a fixed high-intensity light source and a handheld lens and is typically performed by an ophthalmologist.
In addition to diagnosing retinopathies, ophthalmoscopy may be useful in diagnosing Alzheimer disease.[18] Individuals with Alzheimer disease exhibit a higher level of drusen accumulation in their peripheral retinas compared to controls. Although preliminary, this finding could be significant since it could provide a non-invasive method for evaluating Alzheimer disease which is not currently available.
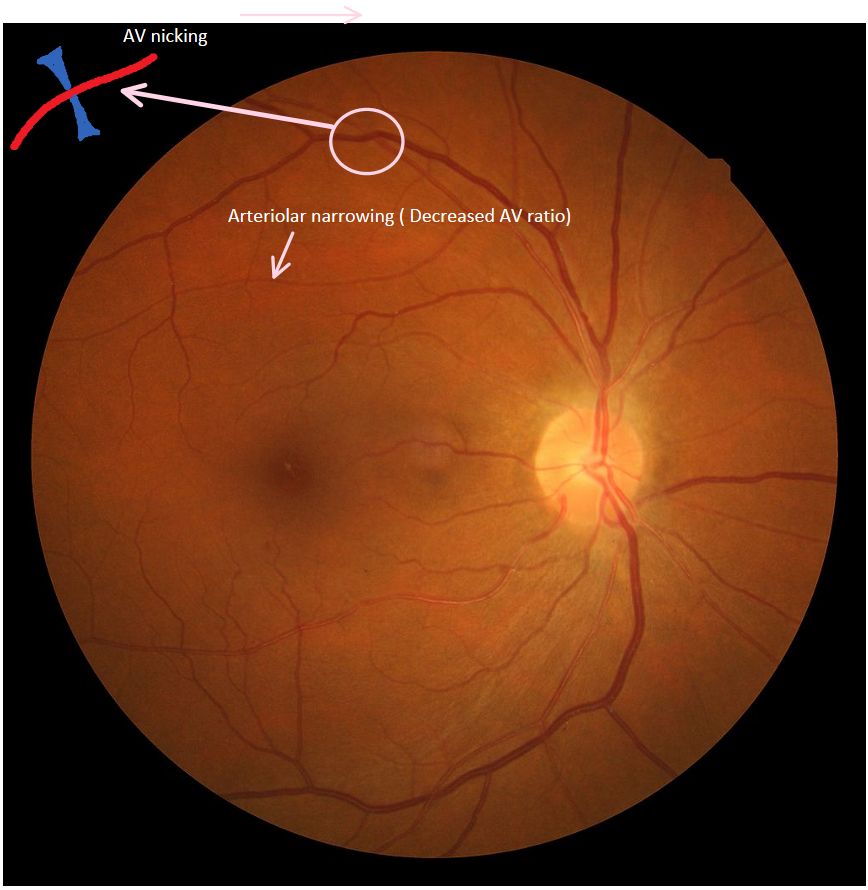
Hypertensive retinopathies are best identified using a standardized examination of fundus photographs as compared to clinical ophthalmoscopy.[17] A variety of staging paradigms for retinopathies have been developed that rely heavily on ophthalmoscopy. The Keith-Wagener-Barker (KWB) system is widely used to grade hypertensive retinopathy. This system divides hypertensive retinopathy into four grades:
- Hypertension with mild narrowing of the retinal arteries and no symptoms present
- Hypertension with more pronounced arterial narrowing and generally no symptoms present
- Overt signs of retinal damage, e.g., retinal hemorrhage, cotton wool spots, “copper wiring” of arterioles and symptoms may be present
- Grade 3 criteria plus papilledema, i.e., swelling of the optic disc and symptoms are present - the symptoms can include vision loss, blurred or double vision, and headaches
The KWB system has come under criticism for not being able to clearly distinguish grade 1 from grade 2 and an alternative system; the Mitchel-Wong system has been proposed with combines the grade 1 and grade 2 of the KWB system into one grade.[19]
As mentioned above, diabetic retinopathy falls into two primary classes, proliferative and non-proliferative. The Scottish grading system is a more elaborate and detailed protocol for grading diabetic retinopathy, and it relies on a digital retinal photograph (27418727). A clinical classification system was developed for AMD that emphasizes drusen accumulation.[20]
Retinopathy of prematurity (ROP) has five stages. Blood vessel growth can be mildly abnormal (stage 1), moderately abnormal (stage 2) or severely abnormal (stage 3 and above). In stage 4 the retina is partially detached, and in stage 5 the retina is completely detached which defines the end stage of this disease.
Treatment / Management
The vast majority of newborns with retinopathy of prematurity have a mild form of the disease (stages 1 and 2), and their condition improves without any treatment. Careful monitoring and delivery of oxygen therapy have reduced oxygen toxicity. About 8 to 10% of newborns with ROP require medical intervention such as laser therapy or cryotherapy.
Lifestyle modifications are usually the first tier of preventative treatment for most of the retinopathies described above. These modifications could include weight loss, dietary changes such as portion control, aerobic exercise (e.g., greater than 130 steps per min), smoking cessation, reduction/elimination of alcohol and maintaining normal lipoprotein-cholesterol levels. For hypertensive retinopathy, excellent, cost-effective hypertensive medications are readily available. For diabetic retinopathy, glycemic control is a crucial factor.[21] For proliferative diabetic retinopathy, exercise requires particular caution due to the increased risk of retinal hemorrhage and retinal detachment. Any exercise that increases blood pressure should be avoided. Metformin, a medication used to treat type 2 diabetes, slows the development of diabetic retinopathy.[22] AMD specific treatments include dietary supplementation with antioxidant nutrients, injection of ranibizumab, implantation of a miniature telescope in patients with end-stage macular degeneration. Ranibizumab is a monoclonal antibody fragment that inhibits VEGF. There are several maternal lifestyle risk factors for premature or low birth weight infants that could be modified to help prevent ROP, e.g., smoking cessation, early care during pregnancy, dealing with substance abuse disorders and minimizing stress.
Differential Diagnosis
- Branch retinal vein occlusion
- Central retinal vein occlusion
- Hemoglobinopathy retinopathy
- Macular edema in diabetes
Enhancing Healthcare Team Outcomes
Patients with vision changes typically have their first encounter with the nurse practitioner, general physician, pediatrician or internist. In each case, it is essential to refer the patient to an ophthalmologist for definitive work up. The role of the primary care providers is to emphasize to the patient the need to stop smoking, maintain healthy body weight, eat a healthy diet and wear sunglasses when going out.
Retinopathy, irrepsective of its underlying cause, is best managed by an interprofessional team combining physicians (both GP and ophthalmologist), and specilaty trained ophthlmology nursing, communicating with the patient and other health team members to bring about optimal patient care and results.