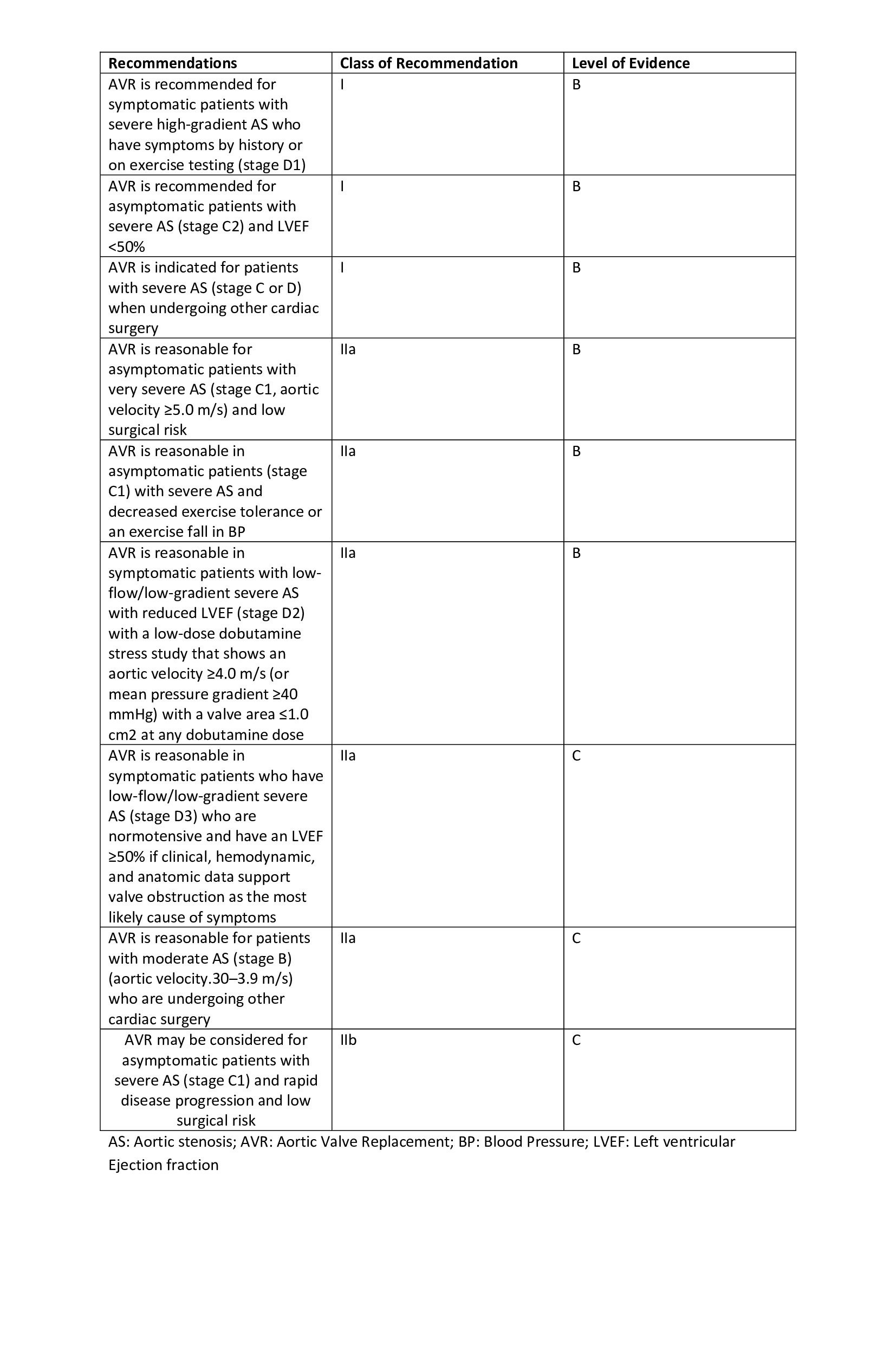
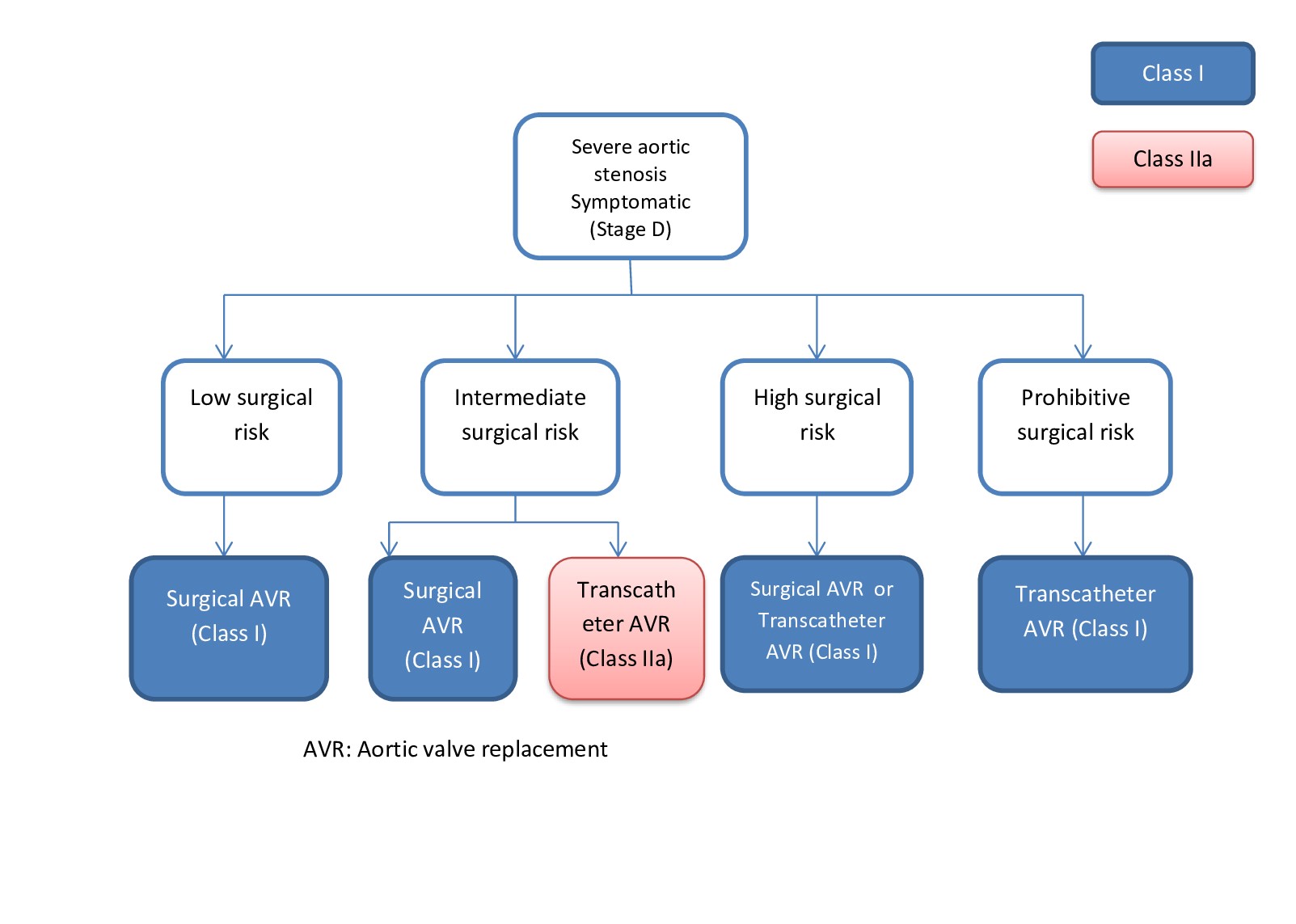
[1]
Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O'Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017 Jun 20:135(25):e1159-e1195. doi: 10.1161/CIR.0000000000000503. Epub 2017 Mar 15
[PubMed PMID: 28298458]
Level 1 (high-level) evidence
[2]
Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM 3rd, Thomas JD, ACC/AHA Task Force Members. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014 Jun 10:129(23):2440-92. doi: 10.1161/CIR.0000000000000029. Epub 2014 Mar 3
[PubMed PMID: 24589852]
Level 1 (high-level) evidence
[4]
Shultz BN, Timek T, Davis AT, Heiser J, Murphy E, Willekes C, Hooker R. A propensity matched analysis of outcomes and long term survival in stented versus stentless valves. Journal of cardiothoracic surgery. 2017 May 31:12(1):45. doi: 10.1186/s13019-017-0608-2. Epub 2017 May 31
[PubMed PMID: 28569201]
[5]
Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL, ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. European heart journal. 2017 Sep 21:38(36):2739-2791. doi: 10.1093/eurheartj/ehx391. Epub
[PubMed PMID: 28886619]
[6]
Dagenais F, Cartier P, Voisine P, Desaulniers D, Perron J, Baillot R, Raymond G, Métras J, Doyle D, Mathieu P. Which biologic valve should we select for the 45- to 65-year-old age group requiring aortic valve replacement? The Journal of thoracic and cardiovascular surgery. 2005 May:129(5):1041-9
[PubMed PMID: 15867778]
[7]
Silver MA, Roberts WC. Detailed anatomy of the normally functioning aortic valve in hearts of normal and increased weight. The American journal of cardiology. 1985 Feb 1:55(4):454-61
[PubMed PMID: 3155899]
[9]
Topan A, Carstina D, Slavcovici A, Rancea R, Capalneanu R, Lupse M. Assesment of the Duke criteria for the diagnosis of infective endocarditis after twenty-years. An analysis of 241 cases. Clujul medical (1957). 2015:88(3):321-6. doi: 10.15386/cjmed-469. Epub 2015 Jul 1
[PubMed PMID: 26609264]
Level 3 (low-level) evidence
[10]
Herrmann JL, Stram AR, Brown JW. Ross Procedure: How to Do It and How to Teach It. World journal for pediatric & congenital heart surgery. 2019 Sep:10(5):624-627. doi: 10.1177/2150135119852324. Epub
[PubMed PMID: 31496411]
[11]
Byrne JG, Rezai K, Sanchez JA, Bernstein RA, Okum E, Leacche M, Balaguer JM, Prabhakaran S, Bridges CR, Higgins RS. Surgical management of endocarditis: the society of thoracic surgeons clinical practice guideline. The Annals of thoracic surgery. 2011 Jun:91(6):2012-9. doi: 10.1016/j.athoracsur.2011.01.106. Epub
[PubMed PMID: 21620012]
Level 1 (high-level) evidence
[12]
Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM 3rd, Thomas JD, American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014 Jun 10:63(22):2438-88. doi: 10.1016/j.jacc.2014.02.537. Epub 2014 Mar 3
[PubMed PMID: 24603192]
Level 1 (high-level) evidence
[13]
Luciani GB, Santini F, Mazzucco A. Autografts, homografts, and xenografts: overview on stentless aortic valve surgery. Journal of cardiovascular medicine (Hagerstown, Md.). 2007 Feb:8(2):91-6
[PubMed PMID: 17299289]
Level 3 (low-level) evidence
[14]
Oury JH. Clinical aspects of the Ross procedure: indications and contraindications. Seminars in thoracic and cardiovascular surgery. 1996 Oct:8(4):328-35
[PubMed PMID: 8899918]
[15]
Solari S, Mastrobuoni S, De Kerchove L, Navarra E, Astarci P, Noirhomme P, Poncelet A, Jashari R, Rubay J, El Khoury G. Over 20 years experience with aortic homograft in aortic valve replacement during acute infective endocarditis. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2016 Dec:50(6):1158-1164. doi: 10.1093/ejcts/ezw175. Epub 2016 May 26
[PubMed PMID: 27229671]
[16]
Concha M, Aranda PJ, Casares J, Merino C, Alados P, Muñoz I, Gonzalez JR, Ribes R, Villalba R. The Ross procedure. Journal of cardiac surgery. 2004 Sep-Oct:19(5):401-9
[PubMed PMID: 15383050]
[18]
Reser D, Caliskan E, Tolboom H, Guidotti A, Maisano F. Median sternotomy. Multimedia manual of cardiothoracic surgery : MMCTS. 2015:2015():. pii: mmv017. doi: 10.1093/mmcts/mmv017. Epub 2015 Jul 17
[PubMed PMID: 26188337]
[19]
Conklin LD, Reardon MJ. Technical aspects of the Ross procedure. Texas Heart Institute journal. 2001:28(3):186-9
[PubMed PMID: 11678251]
[20]
Berdajs D. Ross procedure for everyone. Swiss medical weekly. 2012:142():w13641. doi: 10.4414/smw.2012.13641. Epub 2012 Jul 23
[PubMed PMID: 22826114]
[21]
Etnel JRG, Grashuis P, Huygens SA, Pekbay B, Papageorgiou G, Helbing WA, Roos-Hesselink JW, Bogers AJJC, Mokhles MM, Takkenberg JJM. The Ross Procedure: A Systematic Review, Meta-Analysis, and Microsimulation. Circulation. Cardiovascular quality and outcomes. 2018 Dec:11(12):e004748. doi: 10.1161/CIRCOUTCOMES.118.004748. Epub
[PubMed PMID: 30562065]
Level 2 (mid-level) evidence
[22]
Fukushima S, Tesar PJ, Pearse B, Jalali H, Sparks L, Fraser JF, Pohlner PG. Long-term clinical outcomes after aortic valve replacement using cryopreserved aortic allograft. The Journal of thoracic and cardiovascular surgery. 2014 Jul:148(1):65-72.e2. doi: 10.1016/j.jtcvs.2013.07.038. Epub 2013 Sep 8
[PubMed PMID: 24021951]
Level 2 (mid-level) evidence
[23]
Nappi F, Nenna A, Petitti T, Spadaccio C, Gambardella I, Lusini M, Chello M, Acar C. Long-term outcome of cryopreserved allograft for aortic valve replacement. The Journal of thoracic and cardiovascular surgery. 2018 Oct:156(4):1357-1365.e6. doi: 10.1016/j.jtcvs.2018.04.040. Epub 2018 Apr 18
[PubMed PMID: 29759737]
[24]
Sibilitz KL, Berg SK, Rasmussen TB, Risom SS, Thygesen LC, Tang L, Hansen TB, Johansen PP, Gluud C, Lindschou J, Schmid JP, Hassager C, Køber L, Taylor RS, Zwisler AD. Cardiac rehabilitation increases physical capacity but not mental health after heart valve surgery: a randomised clinical trial. Heart (British Cardiac Society). 2016 Dec 15:102(24):1995-2003. doi: 10.1136/heartjnl-2016-309414. Epub 2016 Aug 4
[PubMed PMID: 27492941]
Level 1 (high-level) evidence