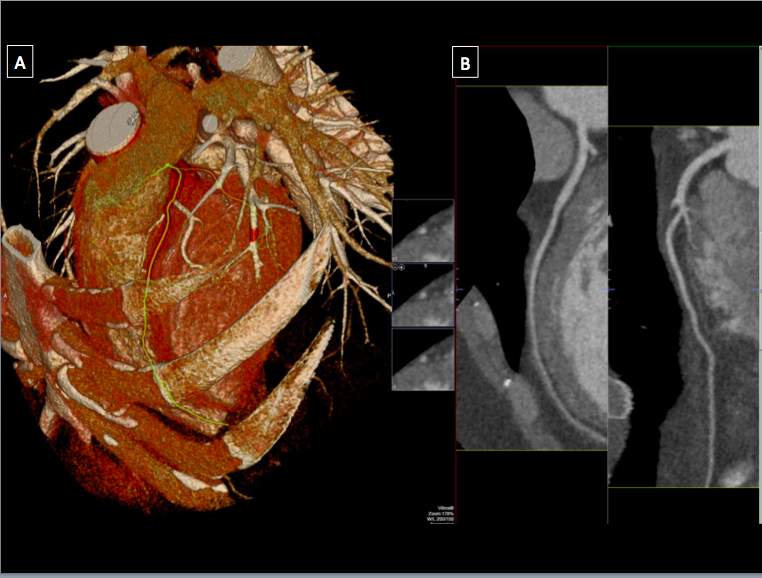
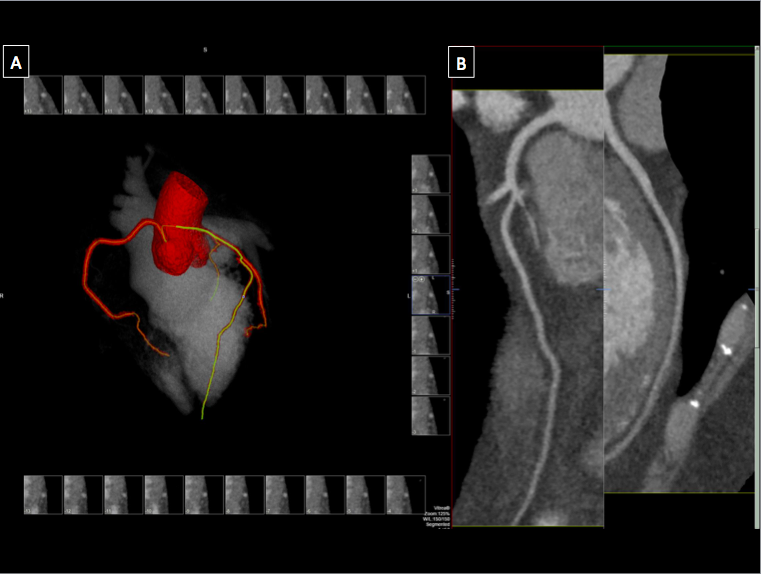
[1]
Eckart RE, Scoville SL, Campbell CL, Shry EA, Stajduhar KC, Potter RN, Pearse LA, Virmani R. Sudden death in young adults: a 25-year review of autopsies in military recruits. Annals of internal medicine. 2004 Dec 7:141(11):829-34
[PubMed PMID: 15583223]
[2]
Young PM, Gerber TC, Williamson EE, Julsrud PR, Herfkens RJ. Cardiac imaging: Part 2, normal, variant, and anomalous configurations of the coronary vasculature. AJR. American journal of roentgenology. 2011 Oct:197(4):816-26. doi: 10.2214/AJR.10.7249. Epub
[PubMed PMID: 21940568]
[3]
Vohra A, Narula H. Dual left anterior descending artery with anomalous origin of long LAD from pulmonary artery - rare coronary anomaly detected on computed tomography coronary angiography. The Indian journal of radiology & imaging. 2016 Apr-Jun:26(2):201-5. doi: 10.4103/0971-3026.184423. Epub
[PubMed PMID: 27413266]
[4]
Wesselhoeft H, Fawcett JS, Johnson AL. Anomalous origin of the left coronary artery from the pulmonary trunk. Its clinical spectrum, pathology, and pathophysiology, based on a review of 140 cases with seven further cases. Circulation. 1968 Aug:38(2):403-25
[PubMed PMID: 5666852]
Level 3 (low-level) evidence
[5]
Surhonne PS, Bogle SR, Bhairappa S, Gupta AK. Percutaneous coronary intervention in a case of anomalous single coronary artery. BMJ case reports. 2016 May 17:2016():. doi: 10.1136/bcr-2016-215651. Epub 2016 May 17
[PubMed PMID: 27190134]
Level 3 (low-level) evidence
[6]
Desmet W, Vanhaecke J, Vrolix M, Van de Werf F, Piessens J, Willems J, de Geest H. Isolated single coronary artery: a review of 50,000 consecutive coronary angiographies. European heart journal. 1992 Dec:13(12):1637-40
[PubMed PMID: 1289093]
[7]
Al-Mohaissen M, Heilbron B, Leipsic J, Ignaszewski A. Anomalous origin of the entire coronary system by three separate ostia within the right coronary sinus--a rarely observed coronary anomaly. The Canadian journal of cardiology. 2010 Jun-Jul:26(6):206-8
[PubMed PMID: 20548983]
[8]
Angelini P. Coronary artery anomalies: an entity in search of an identity. Circulation. 2007 Mar 13:115(10):1296-305
[PubMed PMID: 17353457]
[9]
Kastellanos S, Aznaouridis K, Vlachopoulos C, Tsiamis E, Oikonomou E, Tousoulis D. Overview of coronary artery variants, aberrations and anomalies. World journal of cardiology. 2018 Oct 26:10(10):127-140. doi: 10.4330/wjc.v10.i10.127. Epub
[PubMed PMID: 30386490]
Level 3 (low-level) evidence
[10]
Belostotsky V, Veljanovska L, Hristov N, Mitrev Z. Dual left anterior descending artery distribution. Interactive cardiovascular and thoracic surgery. 2010 Apr:10(4):648-9. doi: 10.1510/icvts.2009.221820. Epub 2010 Jan 15
[PubMed PMID: 20080532]
[11]
Spindola-Franco H, Grose R, Solomon N. Dual left anterior descending coronary artery: angiographic description of important variants and surgical implications. American heart journal. 1983 Mar:105(3):445-55
[PubMed PMID: 6829406]
[12]
Manchanda A, Qureshi A, Brofferio A, Go D, Shirani J. Novel variant of dual left anterior descending coronary artery. Journal of cardiovascular computed tomography. 2010 Mar-Apr:4(2):139-41. doi: 10.1016/j.jcct.2009.12.007. Epub 2010 Jan 14
[PubMed PMID: 20430346]
[13]
Dadkhah-Tirani H, Salari A, Shafighnia S, Hosseini SF, Naghdipoor M. Coronary artery to pulmonary artery fistula. The American journal of case reports. 2013:14():486-8. doi: 10.12659/AJCR.889416. Epub 2013 Nov 18
[PubMed PMID: 24298301]
Level 3 (low-level) evidence
[14]
Barsoum EA, Saiful FB, Asti D, Morcus R, Khoueiry G, Lafferty J, McCord DA. Rare case of coronary to pulmonary vein fistula with coronary steal phenomenon. World journal of cardiology. 2014 Jul 26:6(7):682-4. doi: 10.4330/wjc.v6.i7.682. Epub
[PubMed PMID: 25068029]
Level 3 (low-level) evidence
[15]
Russo FD, Ahmadian HR, Slim AM. Anomalous left anterior descending artery to coronary sinus fistula with associated localized ischemia: A clinical dilemma. The American journal of case reports. 2014:15():107-10. doi: 10.12659/AJCR.890002. Epub 2014 Mar 10
[PubMed PMID: 24644528]
Level 3 (low-level) evidence
[16]
Cakar MA, Tatli E. Coronary-cameral fistula with angina pectoris. Case reports in medicine. 2010:2010():362532. doi: 10.1155/2010/362532. Epub 2010 Dec 6
[PubMed PMID: 21209744]
Level 3 (low-level) evidence
[17]
Furuichi S, Sangiorgi GM, Palloshi A, Godino C, Airoldi F, Montorfano M, Chieffo A, Michev I, Carlino M, Colombo A. Drug-eluting stent implantation in coronary trifurcation lesions. The Journal of invasive cardiology. 2007 Apr:19(4):157-62
[PubMed PMID: 17404400]
[18]
Morales AR, Romanelli R, Tate LG, Boucek RJ, de Marchena E. Intramural left anterior descending coronary artery: significance of the depth of the muscular tunnel. Human pathology. 1993 Jul:24(7):693-701
[PubMed PMID: 8319950]
[19]
Ilia R, Rosenshtein G, Weinstein J, Cafri C, Abu-Ful A, Gueron M. Left anterior descending artery length in left and right coronary artery dominance. Coronary artery disease. 2001 Feb:12(1):77-8
[PubMed PMID: 11211170]
[20]
Kobayashi N, Maehara A, Mintz GS, Wolff SD, Généreux P, Xu K, Mehran R, Gibson CM, Brener SJ, Stone GW. Usefulness of the Left Anterior Descending Artery Wrapping Around the Left Ventricular Apex to Predict Adverse Clinical Outcomes in Patients With Anterior Wall ST-Segment Elevation Myocardial Infarction (an INFUSE-AMI Substudy). The American journal of cardiology. 2015 May 15:115(10):1389-95. doi: 10.1016/j.amjcard.2015.02.034. Epub 2015 Feb 19
[PubMed PMID: 25770973]
Level 2 (mid-level) evidence