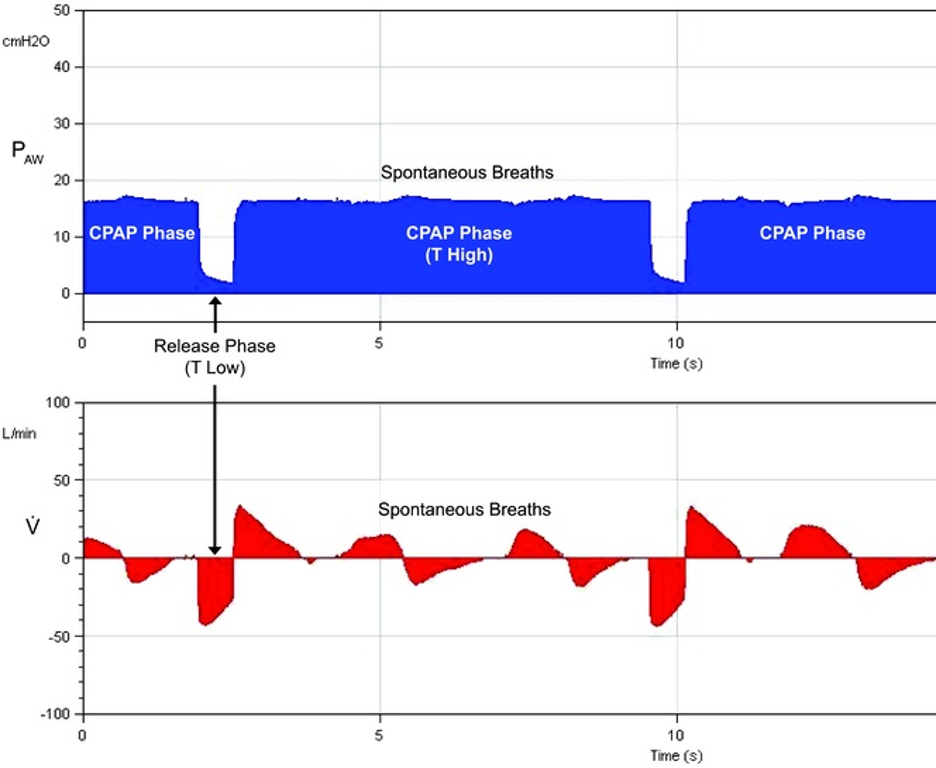
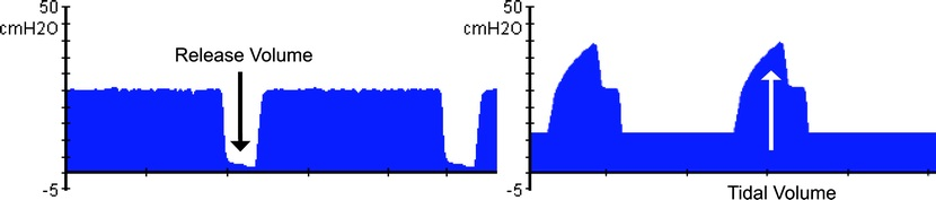
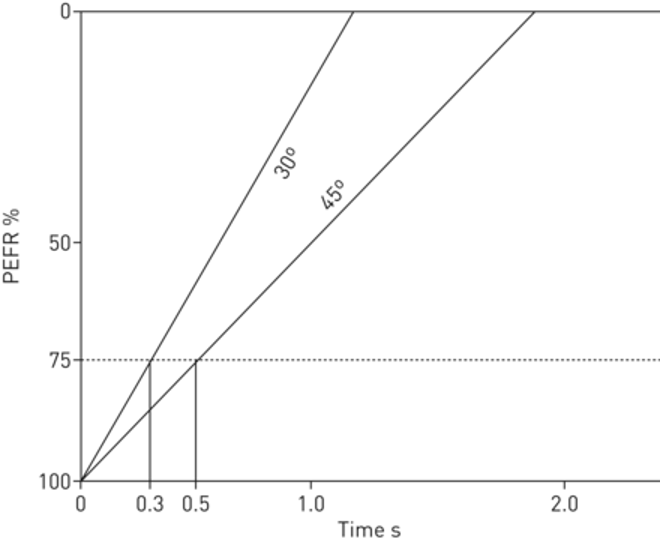
[2]
Pham T, Brochard LJ, Slutsky AS. Mechanical Ventilation: State of the Art. Mayo Clinic proceedings. 2017 Sep:92(9):1382-1400. doi: 10.1016/j.mayocp.2017.05.004. Epub
[PubMed PMID: 28870355]
[3]
Farkas A, Lynch MJ, Westover R, Giles J, Siripong N, Nalatwad A, Pizon AF, Martin-Gill C. Pulmonary Complications of Opioid Overdose Treated With Naloxone. Annals of emergency medicine. 2020 Jan:75(1):39-48. doi: 10.1016/j.annemergmed.2019.04.006. Epub 2019 Jun 8
[PubMed PMID: 31182316]
[4]
Jablonski R, Bhorade S, Strek ME, Dematte J. Recognition and Management of Myositis-Associated Rapidly Progressive Interstitial Lung Disease. Chest. 2020 Jul:158(1):252-263. doi: 10.1016/j.chest.2020.01.033. Epub 2020 Feb 12
[PubMed PMID: 32059958]
[5]
Neumann B, Angstwurm K, Mergenthaler P, Kohler S, Schönenberger S, Bösel J, Neumann U, Vidal A, Huttner HB, Gerner ST, Thieme A, Steinbrecher A, Dunkel J, Roth C, Schneider H, Schimmel E, Fuhrer H, Fahrendorf C, Alberty A, Zinke J, Meisel A, Dohmen C, Stetefeld HR, German Myasthenic Crisis Study Group. Myasthenic crisis demanding mechanical ventilation: A multicenter analysis of 250 cases. Neurology. 2020 Jan 21:94(3):e299-e313. doi: 10.1212/WNL.0000000000008688. Epub 2019 Dec 4
[PubMed PMID: 31801833]
Level 3 (low-level) evidence
[6]
Jung B, Martinez M, Claessens YE, Darmon M, Klouche K, Lautrette A, Levraut J, Maury E, Oberlin M, Terzi N, Viglino D, Yordanov Y, Claret PG, Bigé N, Société de Réanimation de Langue Française (SRLF), Société Française de Médecine d’Urgence (SFMU). Diagnosis and management of metabolic acidosis: guidelines from a French expert panel. Annals of intensive care. 2019 Aug 15:9(1):92. doi: 10.1186/s13613-019-0563-2. Epub 2019 Aug 15
[PubMed PMID: 31418093]
[7]
Cronin JN, Camporota L, Formenti F. Mechanical ventilation in COVID-19: A physiological perspective. Experimental physiology. 2022 Jul:107(7):683-693. doi: 10.1113/EP089400. Epub 2021 Sep 27
[PubMed PMID: 34541721]
Level 3 (low-level) evidence
[9]
Spiegel R, Hockstein M. Airway Pressure Release Ventilation: A Field Guide for the Emergency Physician. Emergency medicine clinics of North America. 2022 Aug:40(3):489-501. doi: 10.1016/j.emc.2022.05.004. Epub 2022 Jul 8
[PubMed PMID: 35953213]
[10]
Mireles-Cabodevila E, Hatipoğlu U, Chatburn RL. A rational framework for selecting modes of ventilation. Respiratory care. 2013 Feb:58(2):348-66. doi: 10.4187/respcare.01839. Epub
[PubMed PMID: 22710796]
[11]
Chang HC, Ho CH, Kung SC, Chen WL, Wang CM, Cheng KC, Liu WL, Hsu HS. Maintenance of low driving pressure in patients with early acute respiratory distress syndrome significantly affects outcomes. Respiratory research. 2021 Dec 15:22(1):313. doi: 10.1186/s12931-021-01912-8. Epub 2021 Dec 15
[PubMed PMID: 34911557]
[12]
Laffey JG, O'Croinin D, McLoughlin P, Kavanagh BP. Permissive hypercapnia--role in protective lung ventilatory strategies. Intensive care medicine. 2004 Mar:30(3):347-56
[PubMed PMID: 14722644]
[13]
Girardis M, Busani S, Damiani E, Donati A, Rinaldi L, Marudi A, Morelli A, Antonelli M, Singer M. Effect of Conservative vs Conventional Oxygen Therapy on Mortality Among Patients in an Intensive Care Unit: The Oxygen-ICU Randomized Clinical Trial. JAMA. 2016 Oct 18:316(15):1583-1589. doi: 10.1001/jama.2016.11993. Epub
[PubMed PMID: 27706466]
Level 1 (high-level) evidence
[14]
Palmer E, Post B, Klapaukh R, Marra G, MacCallum NS, Brealey D, Ercole A, Jones A, Ashworth S, Watkinson P, Beale R, Brett SJ, Young JD, Black C, Rashan A, Martin D, Singer M, Harris S. The Association between Supraphysiologic Arterial Oxygen Levels and Mortality in Critically Ill Patients. A Multicenter Observational Cohort Study. American journal of respiratory and critical care medicine. 2019 Dec 1:200(11):1373-1380. doi: 10.1164/rccm.201904-0849OC. Epub
[PubMed PMID: 31513754]
[15]
Krebs J, Pelosi P, Rocco PRM, Hagmann M, Luecke T. Positive end-expiratory pressure titrated according to respiratory system mechanics or to ARDSNetwork table did not guarantee positive end-expiratory transpulmonary pressure in acute respiratory distress syndrome. Journal of critical care. 2018 Dec:48():433-442. doi: 10.1016/j.jcrc.2018.10.005. Epub 2018 Oct 10
[PubMed PMID: 30336419]
[16]
Fredericks AS, Bunker MP, Gliga LA, Ebeling CG, Ringqvist JR, Heravi H, Manley J, Valladares J, Romito BT. Airway Pressure Release Ventilation: A Review of the Evidence, Theoretical Benefits, and Alternative Titration Strategies. Clinical medicine insights. Circulatory, respiratory and pulmonary medicine. 2020:14():1179548420903297. doi: 10.1177/1179548420903297. Epub 2020 Feb 5
[PubMed PMID: 32076372]
[17]
Zhou Y, Jin X, Lv Y, Wang P, Yang Y, Liang G, Wang B, Kang Y. Early application of airway pressure release ventilation may reduce the duration of mechanical ventilation in acute respiratory distress syndrome. Intensive care medicine. 2017 Nov:43(11):1648-1659. doi: 10.1007/s00134-017-4912-z. Epub 2017 Sep 22
[PubMed PMID: 28936695]
[18]
Kollisch-Singule M, Andrews P, Satalin J, Gatto LA, Nieman GF, Habashi NM. The time-controlled adaptive ventilation protocol: mechanistic approach to reducing ventilator-induced lung injury. European respiratory review : an official journal of the European Respiratory Society. 2019 Jun 30:28(152):. doi: 10.1183/16000617.0126-2018. Epub 2019 Apr 17
[PubMed PMID: 30996041]
[19]
Habashi NM. Other approaches to open-lung ventilation: airway pressure release ventilation. Critical care medicine. 2005 Mar:33(3 Suppl):S228-40
[PubMed PMID: 15753733]
[20]
Fuller BM, Mohr NM, Ablordeppey E, Roman O, Mittauer D, Yan Y, Kollef MH, Carpenter CR, Roberts BW. The Practice Change and Clinical Impact of Lung-Protective Ventilation Initiated in the Emergency Department: A Secondary Analysis of Individual Patient-Level Data From Prior Clinical Trials and Cohort Studies. Critical care medicine. 2023 Feb 1:51(2):279-290. doi: 10.1097/CCM.0000000000005717. Epub 2022 Nov 14
[PubMed PMID: 36374044]
[21]
Weinberger J, Cocoros N, Klompas M. Ventilator-Associated Events: Epidemiology, Risk Factors, and Prevention. Infectious disease clinics of North America. 2021 Dec:35(4):871-899. doi: 10.1016/j.idc.2021.07.005. Epub
[PubMed PMID: 34752224]
[22]
Klompas M. Ventilator-Associated Events: What They Are and What They Are Not. Respiratory care. 2019 Aug:64(8):953-961. doi: 10.4187/respcare.07059. Epub
[PubMed PMID: 31346070]
[23]
Qi W, Murphy TE, Doyle MM, Ferrante LE. Association Between Daily Average of Mobility Achieved During Physical Therapy Sessions and Hospital-Acquired or Ventilator-Associated Pneumonia among Critically Ill Patients. Journal of intensive care medicine. 2023 May:38(5):418-424. doi: 10.1177/08850666221133318. Epub 2022 Oct 22
[PubMed PMID: 36278257]
Level 2 (mid-level) evidence
[24]
Hassan EA, Elsaman SEA. Relationship between ventilator bundle compliance and the occurrence of ventilator-associated events: a prospective cohort study. BMC nursing. 2022 Aug 1:21(1):207. doi: 10.1186/s12912-022-00997-w. Epub 2022 Aug 1
[PubMed PMID: 35915444]
[25]
Grübler MR, Wigger O, Berger D, Blöchlinger S. Basic concepts of heart-lung interactions during mechanical ventilation. Swiss medical weekly. 2017:147():w14491. doi: 10.4414/smw.2017.14491. Epub 2017 Sep 12
[PubMed PMID: 28944931]
[26]
Sutherasan Y, Vargas M, Pelosi P. Protective mechanical ventilation in the non-injured lung: review and meta-analysis. Critical care (London, England). 2014 Mar 18:18(2):211. doi: 10.1186/cc13778. Epub 2014 Mar 18
[PubMed PMID: 24762100]
Level 1 (high-level) evidence
[27]
Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The New England journal of medicine. 2000 May 4:342(18):1301-8
[PubMed PMID: 10793162]
[28]
Needham DM, Colantuoni E, Mendez-Tellez PA, Dinglas VD, Sevransky JE, Dennison Himmelfarb CR, Desai SV, Shanholtz C, Brower RG, Pronovost PJ. Lung protective mechanical ventilation and two year survival in patients with acute lung injury: prospective cohort study. BMJ (Clinical research ed.). 2012 Apr 5:344():e2124. doi: 10.1136/bmj.e2124. Epub 2012 Apr 5
[PubMed PMID: 22491953]
[29]
Mosier JM, Hypes C, Joshi R, Whitmore S, Parthasarathy S, Cairns CB. Ventilator Strategies and Rescue Therapies for Management of Acute Respiratory Failure in the Emergency Department. Annals of emergency medicine. 2015 Nov:66(5):529-41. doi: 10.1016/j.annemergmed.2015.04.030. Epub 2015 May 23
[PubMed PMID: 26014437]
[30]
Weingart SD. Managing Initial Mechanical Ventilation in the Emergency Department. Annals of emergency medicine. 2016 Nov:68(5):614-617. doi: 10.1016/j.annemergmed.2016.04.059. Epub 2016 Jun 9
[PubMed PMID: 27289336]
[31]
Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT, National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. The New England journal of medicine. 2004 Jul 22:351(4):327-36
[PubMed PMID: 15269312]
[32]
Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat A, Richard JC, Carvalho CR, Brower RG. Driving pressure and survival in the acute respiratory distress syndrome. The New England journal of medicine. 2015 Feb 19:372(8):747-55. doi: 10.1056/NEJMsa1410639. Epub
[PubMed PMID: 25693014]
[33]
Fuchs H, Rossmann N, Schmid MB, Hoenig M, Thome U, Mayer B, Klotz D, Hummler HD. Permissive hypercapnia for severe acute respiratory distress syndrome in immunocompromised children: A single center experience. PloS one. 2017:12(6):e0179974. doi: 10.1371/journal.pone.0179974. Epub 2017 Jun 20
[PubMed PMID: 28632754]
[34]
Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) Investigators, Cavalcanti AB, Suzumura ÉA, Laranjeira LN, Paisani DM, Damiani LP, Guimarães HP, Romano ER, Regenga MM, Taniguchi LNT, Teixeira C, Pinheiro de Oliveira R, Machado FR, Diaz-Quijano FA, Filho MSA, Maia IS, Caser EB, Filho WO, Borges MC, Martins PA, Matsui M, Ospina-Tascón GA, Giancursi TS, Giraldo-Ramirez ND, Vieira SRR, Assef MDGPL, Hasan MS, Szczeklik W, Rios F, Amato MBP, Berwanger O, Ribeiro de Carvalho CR. Effect of Lung Recruitment and Titrated Positive End-Expiratory Pressure (PEEP) vs Low PEEP on Mortality in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA. 2017 Oct 10:318(14):1335-1345. doi: 10.1001/jama.2017.14171. Epub
[PubMed PMID: 28973363]
Level 3 (low-level) evidence
[35]
Writing Group for the PReVENT Investigators, Simonis FD, Serpa Neto A, Binnekade JM, Braber A, Bruin KCM, Determann RM, Goekoop GJ, Heidt J, Horn J, Innemee G, de Jonge E, Juffermans NP, Spronk PE, Steuten LM, Tuinman PR, de Wilde RBP, Vriends M, Gama de Abreu M, Pelosi P, Schultz MJ. Effect of a Low vs Intermediate Tidal Volume Strategy on Ventilator-Free Days in Intensive Care Unit Patients Without ARDS: A Randomized Clinical Trial. JAMA. 2018 Nov 13:320(18):1872-1880. doi: 10.1001/jama.2018.14280. Epub
[PubMed PMID: 30357256]
Level 1 (high-level) evidence
[36]
Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM, Coursin DB, Herr DL, Tung A, Robinson BR, Fontaine DK, Ramsay MA, Riker RR, Sessler CN, Pun B, Skrobik Y, Jaeschke R, American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Critical care medicine. 2013 Jan:41(1):263-306. doi: 10.1097/CCM.0b013e3182783b72. Epub
[PubMed PMID: 23269131]
Level 1 (high-level) evidence
[37]
Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJC, Pandharipande PP, Watson PL, Weinhouse GL, Nunnally ME, Rochwerg B, Balas MC, van den Boogaard M, Bosma KJ, Brummel NE, Chanques G, Denehy L, Drouot X, Fraser GL, Harris JE, Joffe AM, Kho ME, Kress JP, Lanphere JA, McKinley S, Neufeld KJ, Pisani MA, Payen JF, Pun BT, Puntillo KA, Riker RR, Robinson BRH, Shehabi Y, Szumita PM, Winkelman C, Centofanti JE, Price C, Nikayin S, Misak CJ, Flood PD, Kiedrowski K, Alhazzani W. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Critical care medicine. 2018 Sep:46(9):e825-e873. doi: 10.1097/CCM.0000000000003299. Epub
[PubMed PMID: 30113379]
Level 1 (high-level) evidence
[38]
Wang L, Li X, Yang Z, Tang X, Yuan Q, Deng L, Sun X. Semi-recumbent position versus supine position for the prevention of ventilator-associated pneumonia in adults requiring mechanical ventilation. The Cochrane database of systematic reviews. 2016 Jan 8:2016(1):CD009946. doi: 10.1002/14651858.CD009946.pub2. Epub 2016 Jan 8
[PubMed PMID: 26743945]
Level 1 (high-level) evidence
[39]
Tran A, Fernando SM, Rochwerg B, Cook DJ, Crowther MA, Fowler RA, Alhazzani W, Siegal DM, Castellucci LA, Zarychanski R, English SW, Kyeremanteng K, Carrier M. Prognostic Factors Associated With Development of Venous Thromboembolism in Critically Ill Patients-A Systematic Review and Meta-Analysis. Critical care medicine. 2022 Apr 1:50(4):e370-e381. doi: 10.1097/CCM.0000000000005382. Epub
[PubMed PMID: 34636806]
Level 1 (high-level) evidence
[40]
Krag M, Perner A, Wetterslev J, Wise MP, Borthwick M, Bendel S, McArthur C, Cook D, Nielsen N, Pelosi P, Keus F, Guttormsen AB, Moller AD, Møller MH, SUP-ICU co-authors. Prevalence and outcome of gastrointestinal bleeding and use of acid suppressants in acutely ill adult intensive care patients. Intensive care medicine. 2015 May:41(5):833-45. doi: 10.1007/s00134-015-3725-1. Epub 2015 Apr 10
[PubMed PMID: 25860444]
[41]
Cook DJ, Fuller HD, Guyatt GH, Marshall JC, Leasa D, Hall R, Winton TL, Rutledge F, Todd TJ, Roy P. Risk factors for gastrointestinal bleeding in critically ill patients. Canadian Critical Care Trials Group. The New England journal of medicine. 1994 Feb 10:330(6):377-81
[PubMed PMID: 8284001]
[42]
Alhazzani W, Alshamsi F, Belley-Cote E, Heels-Ansdell D, Brignardello-Petersen R, Alquraini M, Perner A, Møller MH, Krag M, Almenawer S, Rochwerg B, Dionne J, Jaeschke R, Alshahrani M, Deane A, Perri D, Thebane L, Al-Omari A, Finfer S, Cook D, Guyatt G. Efficacy and safety of stress ulcer prophylaxis in critically ill patients: a network meta-analysis of randomized trials. Intensive care medicine. 2018 Jan:44(1):1-11. doi: 10.1007/s00134-017-5005-8. Epub 2017 Dec 4
[PubMed PMID: 29199388]
Level 1 (high-level) evidence
[43]
Cook D, Guyatt G. Prophylaxis against Upper Gastrointestinal Bleeding in Hospitalized Patients. The New England journal of medicine. 2018 Jun 28:378(26):2506-2516. doi: 10.1056/NEJMra1605507. Epub
[PubMed PMID: 29949497]
[44]
Kollef MH. Evaluating the Value of the Respiratory Therapist: Where Is the Evidence? Focus on the Barnes-Jewish Hospital Experience. Respiratory care. 2017 Dec:62(12):1602-1610. doi: 10.4187/respcare.05807. Epub
[PubMed PMID: 29162728]