Introduction
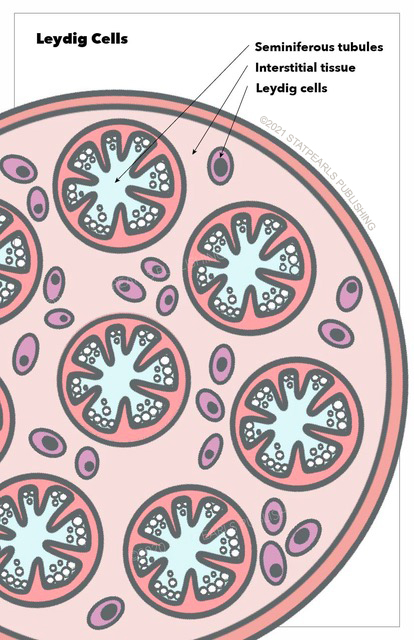
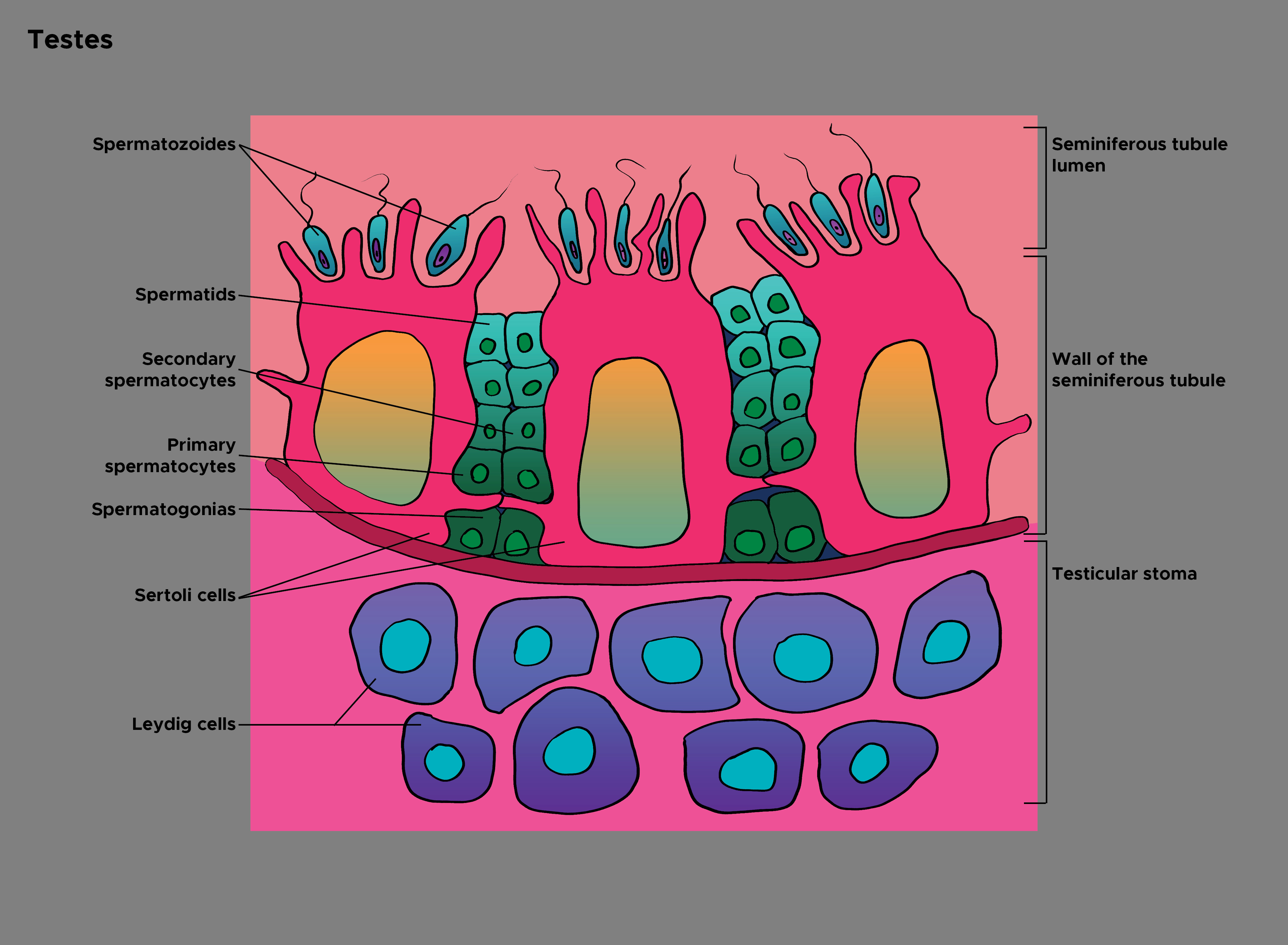
Leydig cells are essential and crucial cells located in the testes of the male gonads. They are known as testicular interstitial cells and can be found between seminiferous tubules, which contain Sertoli and germ cells. Together, these three cell types maintain spermatogenesis, control hormonal regulation, and affect secondary sexual characteristics in males. Leydig cells are under hormonal regulation by the hypothalamic-pituitary axis. These cells can be associated with some pathologic changes or cancerous malformation.[1] See Illustration. Leydig Cells.
Structure
Leydig cells can be found around seminiferous tubules forming groups of up to ten cells. They are generally described as polygonal cells with eosinophilic cytoplasm and a large round nucleus with a prominent nucleolus. Lipid content is abundant.
Because these cells are responsible for testosterone production, they have features of steroid-secreting cells, including large and well developed smooth endoplasmic reticulum, large and numerous lipid droplets, and significant numbers of mitochondria with tubulovesicular cristae. Another common finding in Leydig cells is lipofuscin, which appears as multiple rounded irregular bodies, representing accumulated lipid droplets in lysosomes.
Reinke’s crystalloids are pale-staining cytoplasmic inclusions found specifically in Leydig cells. These crystals have a rod-like or cylindrical shape and are usually arranged in a linear pattern. No function is yet known for these bodies, but some studies suggest they are by-products of testosterone production and steroid metabolism in testes.[1][2][3]
Function
Leydig cells are the primary source of testosterone or androgens in males. This physiology allows them to play a crucial role in many vital physiological processes in males, including sperm production or spermatogenesis, controlling sexual development, and maintaining secondary sexual characteristics and behaviors.
These crucial functions start with androgen production inside Leydig cells; this process is under the regulation and control of many factors, mainly luteinizing hormone (LH), a hormone produced from the pituitary gland. Abundant lipid content and well developed smooth endoplasmic reticulum are features that make Leydig cells more efficient in steroid (androgen) production.[4]
With developments of male embryos, Leydig cells produce androgens inducing Wolffian duct development into male urogenital structures. Sertoli cells produce anti-Mullerian hormone preventing the development of female genital structures. In adults, infantile Leydig cells are replaced by adult Leydig cells maintaining androgen through life.
As mentioned before, Leydig cells exist in the interstitium outside seminiferous tubules, where spermatogenesis occurs. Androgens diffuse from the interstitium into seminiferous tubules to act on germ and Sertoli cells, inducing and maintaining sperm production (see Illustration. Histology of the Testes). It is worth noting that testosterone levels are higher inside testes compared to the serum. Decreased intratesticular testosterone level is associated with defects in spermatogenesis; this has demonstrated links to decreased expression of essential proteins involved in germ cell regulation.[5]
Histochemistry and Cytochemistry
Leydig Cells can be studied or observed using a variety of stains or methods. Hematoxylin-Eosin (HE) staining is one of the most commonly used methods in the field. When staining a testicular specimen using HE stain, Leydig cells can be easily differentiated or isolated because they stain deeper than cells in seminiferous tubules. They appear as oval cells with big and round nuclei.
HSD3B staining is another possibility. 3β-Hydroxysteroid dehydrogenase (HSD3B) is an enzyme that catalyzes the biosynthesis of the steroid progesterone in Leydig cells, making it a specific stain because this enzyme is not present in other testicular cells. Leydig cells show different populations when stained with HSD3B depending on the uptake intensity, which is affected by the lipid content of cells.
Reverse transcriptase-polymerase chain reaction (RT-PCR) can also be beneficial by detecting the transcription of important enzymes in Leydig cells, including HSD3B6, CYP17A1, and StAR.[6]
Microscopy, Light
Light microscopy observation of Leydig cells reveals large polygonal cells with eosinophilic, non-granular cytoplasm, and large round nuclei with prominent nucleoli. The location of the nuclei is mostly at the center of the cytoplasm. The cytoplasm is filled with abundant lipid droplets that have a strong refractivity under light microscopes.[6][7]
Microscopy, Electron
When observed by an electron microscope, Leydig cells are clustered in small numbers forming cords, and they share a thin common basal lamina. Adjacent cells have small canaliculi between them, characterized by rudimentary desmosomes and microvillus processes. Nuclei appear as rounded with clumps of chromatin and prominent nucleoli. The most prominent organelle is the agranular or smooth endoplasmic reticulum. Microscopic examination also reveals many mitochondria, which are elongated and have clear tubular cristae.[7]
Clinical Significance
Leydig cell tumor is a rare subtype of testicular tumors, which are common in young males. This cancer follows a benign course most of the time with rare metastasis. The usual presentation is a painless testicular mass in a young adult; precocious puberty is possible in children. Imaging studies, including ultrasound and CT scans, are important for evaluation, but there are no distinctive ultrasound features to distinguish this tumor. An accurate diagnosis is possible using histology and the identification of Leydig cells and their features, including Reinke's crystals. Standard therapy is radical orchidectomy.
This tumor can occur in females as a subtype of ovarian cancer and can cause virilization.[8][9]
One of the most common pathologies of Leydig cells is Klinefelter syndrome, where testosterone levels are usually low despite high levels of luteinizing hormone and abundant Leydig cells. The cells are abnormal, but no steroid precursor accumulation is noted, which excludes enzyme defects or deficiencies.
Leydig cells are also affected by systemic diseases, including severe renal failure, in these cases, testosterone levels are low, but luteinizing hormone levels will measure as high.[10]