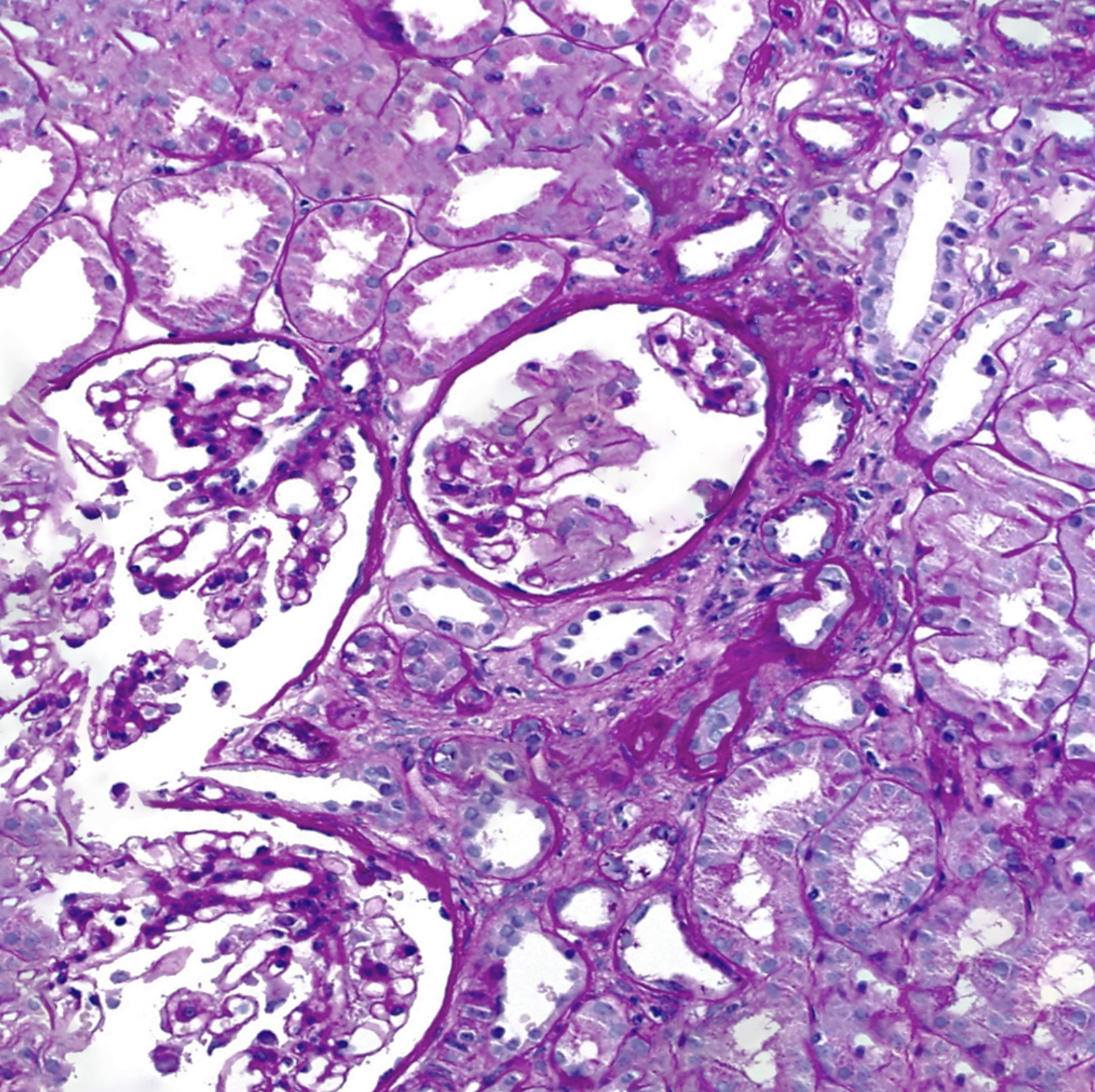
[1]
Zhang Y, Ding J. Renal, auricular, and ocular outcomes of Alport syndrome and their current management. Pediatric nephrology (Berlin, Germany). 2018 Aug:33(8):1309-1316. doi: 10.1007/s00467-017-3784-3. Epub 2017 Sep 1
[PubMed PMID: 28864840]
[3]
Kashtan C. Alport syndrome: facts and opinions. F1000Research. 2017:6():50. doi: 10.12688/f1000research.9636.1. Epub 2017 Jan 17
[PubMed PMID: 28163907]
Level 3 (low-level) evidence
[4]
Savige J. Alport syndrome: deducing the mode of inheritance from the presence of haematuria in family members. Pediatric nephrology (Berlin, Germany). 2020 Jan:35(1):59-66. doi: 10.1007/s00467-018-4121-1. Epub 2018 Nov 30
[PubMed PMID: 30506145]
[5]
Kashtan CE. Renal transplantation in patients with Alport syndrome: patient selection, outcomes, and donor evaluation. International journal of nephrology and renovascular disease. 2018:11():267-270. doi: 10.2147/IJNRD.S150539. Epub 2018 Oct 16
[PubMed PMID: 30410383]
[6]
Vos P, Zietse R, van Geel M, Brooks AS, Cransberg K. Diagnosing Alport Syndrome: Lessons from the Pediatric Ward. Nephron. 2018:140(3):203-210. doi: 10.1159/000492438. Epub 2018 Sep 13
[PubMed PMID: 30212818]
[7]
Katsuma A, Nakada Y, Yamamoto I, Horita S, Furusawa M, Unagami K, Katsumata H, Okumi M, Ishida H, Yokoo T, Tanabe K, Japan Academic Consortium of Kidney Transplantation (JACK). Long-term survival in Japanese renal transplant recipients with Alport syndrome: a retrospective study. BMC nephrology. 2018 Oct 3:19(1):249. doi: 10.1186/s12882-018-1052-9. Epub 2018 Oct 3
[PubMed PMID: 30285655]
Level 2 (mid-level) evidence
[8]
Crockett DK, Pont-Kingdon G, Gedge F, Sumner K, Seamons R, Lyon E. The Alport syndrome COL4A5 variant database. Human mutation. 2010 Aug:31(8):E1652-7. doi: 10.1002/humu.21312. Epub
[PubMed PMID: 20574986]
[9]
Hashimura Y, Nozu K, Kaito H, Nakanishi K, Fu XJ, Ohtsubo H, Hashimoto F, Oka M, Ninchoji T, Ishimori S, Morisada N, Matsunoshita N, Kamiyoshi N, Yoshikawa N, Iijima K. Milder clinical aspects of X-linked Alport syndrome in men positive for the collagen IV α5 chain. Kidney international. 2014 May:85(5):1208-13. doi: 10.1038/ki.2013.479. Epub 2013 Dec 4
[PubMed PMID: 24304881]
[10]
Nozu K, Minamikawa S, Yamada S, Oka M, Yanagita M, Morisada N, Fujinaga S, Nagano C, Gotoh Y, Takahashi E, Morishita T, Yamamura T, Ninchoji T, Kaito H, Morioka I, Nakanishi K, Vorechovsky I, Iijima K. Characterization of contiguous gene deletions in COL4A6 and COL4A5 in Alport syndrome-diffuse leiomyomatosis. Journal of human genetics. 2017 Jul:62(7):733-735. doi: 10.1038/jhg.2017.28. Epub 2017 Mar 9
[PubMed PMID: 28275241]
[11]
Hicks J, Mierau G, Wartchow E, Eldin K. Renal diseases associated with hematuria in children and adolescents: a brief tutorial. Ultrastructural pathology. 2012 Feb:36(1):1-18. doi: 10.3109/01913123.2011.620731. Epub
[PubMed PMID: 22292732]
[12]
Warady BA, Agarwal R, Bangalore S, Chapman A, Levin A, Stenvinkel P, Toto RD, Chertow GM. Alport Syndrome Classification and Management. Kidney medicine. 2020 Sep-Oct:2(5):639-649. doi: 10.1016/j.xkme.2020.05.014. Epub 2020 Aug 7
[PubMed PMID: 33094278]
[13]
Savige J, Colville D, Rheault M, Gear S, Lennon R, Lagas S, Finlay M, Flinter F. Alport Syndrome in Women and Girls. Clinical journal of the American Society of Nephrology : CJASN. 2016 Sep 7:11(9):1713-1720. doi: 10.2215/CJN.00580116. Epub 2016 Jun 10
[PubMed PMID: 27287265]
[14]
Deltas C, Pierides A, Voskarides K. Molecular genetics of familial hematuric diseases. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2013 Dec:28(12):2946-60. doi: 10.1093/ndt/gft253. Epub 2013 Sep 17
[PubMed PMID: 24046192]
[15]
Fallerini C, Dosa L, Tita R, Del Prete D, Feriozzi S, Gai G, Clementi M, La Manna A, Miglietti N, Mancini R, Mandrile G, Ghiggeri GM, Piaggio G, Brancati F, Diano L, Frate E, Pinciaroli AR, Giani M, Castorina P, Bresin E, Giachino D, De Marchi M, Mari F, Bruttini M, Renieri A, Ariani F. Unbiased next generation sequencing analysis confirms the existence of autosomal dominant Alport syndrome in a relevant fraction of cases. Clinical genetics. 2014 Sep:86(3):252-7. doi: 10.1111/cge.12258. Epub 2013 Oct 17
[PubMed PMID: 24033287]
Level 3 (low-level) evidence
[16]
Morinière V, Dahan K, Hilbert P, Lison M, Lebbah S, Topa A, Bole-Feysot C, Pruvost S, Nitschke P, Plaisier E, Knebelmann B, Macher MA, Noel LH, Gubler MC, Antignac C, Heidet L. Improving mutation screening in familial hematuric nephropathies through next generation sequencing. Journal of the American Society of Nephrology : JASN. 2014 Dec:25(12):2740-51. doi: 10.1681/ASN.2013080912. Epub 2014 May 22
[PubMed PMID: 24854265]
[17]
Delimont D, Dufek BM, Meehan DT, Zallocchi M, Gratton MA, Phillips G, Cosgrove D. Laminin α2-mediated focal adhesion kinase activation triggers Alport glomerular pathogenesis. PloS one. 2014:9(6):e99083. doi: 10.1371/journal.pone.0099083. Epub 2014 Jun 10
[PubMed PMID: 24915008]
[18]
Dufek B, Meehan DT, Delimont D, Cheung L, Gratton MA, Phillips G, Song W, Liu S, Cosgrove D. Endothelin A receptor activation on mesangial cells initiates Alport glomerular disease. Kidney international. 2016 Aug:90(2):300-310. doi: 10.1016/j.kint.2016.02.018. Epub 2016 Apr 27
[PubMed PMID: 27165837]
[19]
Gunwar S, Ballester F, Noelken ME, Sado Y, Ninomiya Y, Hudson BG. Glomerular basement membrane. Identification of a novel disulfide-cross-linked network of alpha3, alpha4, and alpha5 chains of type IV collagen and its implications for the pathogenesis of Alport syndrome. The Journal of biological chemistry. 1998 Apr 10:273(15):8767-75
[PubMed PMID: 9535854]
Level 3 (low-level) evidence
[20]
Cosgrove D, Rodgers K, Meehan D, Miller C, Bovard K, Gilroy A, Gardner H, Kotelianski V, Gotwals P, Amatucci A, Kalluri R. Integrin alpha1beta1 and transforming growth factor-beta1 play distinct roles in alport glomerular pathogenesis and serve as dual targets for metabolic therapy. The American journal of pathology. 2000 Nov:157(5):1649-59
[PubMed PMID: 11073824]
[21]
Cosgrove D. Glomerular pathology in Alport syndrome: a molecular perspective. Pediatric nephrology (Berlin, Germany). 2012 Jun:27(6):885-90. doi: 10.1007/s00467-011-1868-z. Epub 2011 Apr 1
[PubMed PMID: 21455721]
Level 3 (low-level) evidence
[22]
Olaru F, Luo W, Wang XP, Ge L, Hertz JM, Kashtan CE, Sado Y, Segal Y, Hudson BG, Borza DB. Quaternary epitopes of α345(IV) collagen initiate Alport post-transplant anti-GBM nephritis. Journal of the American Society of Nephrology : JASN. 2013 May:24(6):889-95. doi: 10.1681/ASN.2012100978. Epub 2013 Apr 25
[PubMed PMID: 23620401]
[23]
Wang XP, Fogo AB, Colon S, Giannico G, Abul-Ezz SR, Miner JH, Borza DB. Distinct epitopes for anti-glomerular basement membrane alport alloantibodies and goodpasture autoantibodies within the noncollagenous domain of alpha3(IV) collagen: a janus-faced antigen. Journal of the American Society of Nephrology : JASN. 2005 Dec:16(12):3563-71
[PubMed PMID: 16236801]
[24]
Borza DB. Autoepitopes and alloepitopes of type IV collagen: role in the molecular pathogenesis of anti-GBM antibody glomerulonephritis. Nephron. Experimental nephrology. 2007:106(2):e37-43
[PubMed PMID: 17570938]
[25]
Kashtan CE. Alport syndrome and thin glomerular basement membrane disease. Journal of the American Society of Nephrology : JASN. 1998 Sep:9(9):1736-50
[PubMed PMID: 9727383]
[26]
Thorner PS. Alport syndrome and thin basement membrane nephropathy. Nephron. Clinical practice. 2007:106(2):c82-8
[PubMed PMID: 17570934]
[27]
Patey-Mariaud de Serre N, Garfa M, Bessiéres B, Noël LH, Knebelmann B. Collagen alpha5 and alpha2(IV) chain coexpression: analysis of skin biopsies of Alport patients. Kidney international. 2007 Aug:72(4):512-6
[PubMed PMID: 17554254]
[28]
Gubler MC. Diagnosis of Alport syndrome without biopsy? Pediatric nephrology (Berlin, Germany). 2007 May:22(5):621-5
[PubMed PMID: 17143627]
[29]
Gubler M, Levy M, Broyer M, Naizot C, Gonzales G, Perrin D, Habib R. Alport's syndrome. A report of 58 cases and a review of the literature. The American journal of medicine. 1981 Mar:70(3):493-505
[PubMed PMID: 7211891]
[30]
Izzedine H, Tankere F, Launay-Vacher V, Deray G. Ear and kidney syndromes: molecular versus clinical approach. Kidney international. 2004 Feb:65(2):369-85
[PubMed PMID: 14717907]
[31]
Shaw EA, Colville D, Wang YY, Zhang KW, Dagher H, Fassett R, Guymer R, Savige J. Characterization of the peripheral retinopathy in X-linked and autosomal recessive Alport syndrome. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2007 Jan:22(1):104-8
[PubMed PMID: 17071739]
[32]
Byrne MC, Budisavljevic MN, Fan Z, Self SE, Ploth DW. Renal transplant in patients with Alport's syndrome. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2002 Apr:39(4):769-75
[PubMed PMID: 11920343]
[33]
Perrin D, Jungers P, Grünfeld JP, Delons S, Noël LH, Zenatti C. Perimacular changes in Alport's syndrome. Clinical nephrology. 1980 Apr:13(4):163-7
[PubMed PMID: 7379367]
[34]
Shah SN, Weinberg DV. Giant macular hole in Alport syndrome. Ophthalmic genetics. 2010 Jun:31(2):94-7. doi: 10.3109/13816811003767128. Epub
[PubMed PMID: 20450313]
[35]
Plevová P, Gut J, Janda J. Familial hematuria: A review. Medicina (Kaunas, Lithuania). 2017:53(1):1-10. doi: 10.1016/j.medici.2017.01.002. Epub 2017 Jan 31
[PubMed PMID: 28236514]
[36]
Cosgrove D, Liu S. Collagen IV diseases: A focus on the glomerular basement membrane in Alport syndrome. Matrix biology : journal of the International Society for Matrix Biology. 2017 Jan:57-58():45-54. doi: 10.1016/j.matbio.2016.08.005. Epub 2016 Aug 27
[PubMed PMID: 27576055]
[37]
Citirik M, Batman C, Men G, Tuncel M, Zilelioglu O. Electron microscopic examination of the anterior lens capsule in a case of Alport's syndrome. Clinical & experimental optometry. 2007 Sep:90(5):367-70
[PubMed PMID: 17697183]
Level 3 (low-level) evidence
[38]
Ahmed F, Kamae KK, Jones DJ, Deangelis MM, Hageman GS, Gregory MC, Bernstein PS. Temporal macular thinning associated with X-linked Alport syndrome. JAMA ophthalmology. 2013 Jun:131(6):777-82. doi: 10.1001/jamaophthalmol.2013.1452. Epub
[PubMed PMID: 23572034]
[39]
Raimundo M, Fonseca C, Silva R, Figueira J. Bilateral giant macular holes: A rare manifestation of Alport syndrome. European journal of ophthalmology. 2019 Jan:29(1):NP13-NP16. doi: 10.1177/1120672118781232. Epub 2018 Jun 6
[PubMed PMID: 29873249]
[40]
Uliana V, Marcocci E, Mucciolo M, Meloni I, Izzi C, Manno C, Bruttini M, Mari F, Scolari F, Renieri A, Salviati L. Alport syndrome and leiomyomatosis: the first deletion extending beyond COL4A6 intron 2. Pediatric nephrology (Berlin, Germany). 2011 May:26(5):717-24. doi: 10.1007/s00467-010-1693-9. Epub 2010 Dec 14
[PubMed PMID: 21380622]
[41]
Liapis H, Foster K, Miner JH. Red cell traverse through thin glomerular basement membrane. Kidney international. 2002 Feb:61(2):762-3
[PubMed PMID: 11849422]
[42]
Cheong HI, Kashtan CE, Kim Y, Kleppel MM, Michael AF. Immunohistologic studies of type IV collagen in anterior lens capsules of patients with Alport syndrome. Laboratory investigation; a journal of technical methods and pathology. 1994 Apr:70(4):553-7
[PubMed PMID: 8176894]
[43]
Gyoneva L, Segal Y, Dorfman KD, Barocas VH. Mechanical response of wild-type and Alport murine lens capsules during osmotic swelling. Experimental eye research. 2013 Aug:113():87-91. doi: 10.1016/j.exer.2013.05.008. Epub 2013 May 21
[PubMed PMID: 23707242]
[44]
Savige J, Ariani F, Mari F, Bruttini M, Renieri A, Gross O, Deltas C, Flinter F, Ding J, Gale DP, Nagel M, Yau M, Shagam L, Torra R, Ars E, Hoefele J, Garosi G, Storey H. Expert consensus guidelines for the genetic diagnosis of Alport syndrome. Pediatric nephrology (Berlin, Germany). 2019 Jul:34(7):1175-1189. doi: 10.1007/s00467-018-3985-4. Epub 2018 Jul 9
[PubMed PMID: 29987460]
Level 3 (low-level) evidence
[45]
Rumpelt HJ. Hereditary nephropathy (Alport syndrome): correlation of clinical data with glomerular basement membrane alterations. Clinical nephrology. 1980 May:13(5):203-7
[PubMed PMID: 7398144]
[46]
Gettelfinger JD, Dahl JP. Syndromic Hearing Loss: A Brief Review of Common Presentations and Genetics. Journal of pediatric genetics. 2018 Mar:7(1):1-8. doi: 10.1055/s-0037-1617454. Epub 2018 Jan 4
[PubMed PMID: 29441214]
[47]
Easson A, Walter S. Hearing-impaired young people - a physician's guide . Clinical medicine (London, England). 2017 Dec:17(6):521-524. doi: 10.7861/clinmedicine.17-6-521. Epub
[PubMed PMID: 29196352]
[48]
Gross O, Licht C, Anders HJ, Hoppe B, Beck B, Tönshoff B, Höcker B, Wygoda S, Ehrich JH, Pape L, Konrad M, Rascher W, Dötsch J, Müller-Wiefel DE, Hoyer P, Study Group Members of the Gesellschaft für Pädiatrische Nephrologie, Knebelmann B, Pirson Y, Grunfeld JP, Niaudet P, Cochat P, Heidet L, Lebbah S, Torra R, Friede T, Lange K, Müller GA, Weber M. Early angiotensin-converting enzyme inhibition in Alport syndrome delays renal failure and improves life expectancy. Kidney international. 2012 Mar:81(5):494-501. doi: 10.1038/ki.2011.407. Epub 2011 Dec 14
[PubMed PMID: 22166847]
[49]
Daina E, Cravedi P, Alpa M, Roccatello D, Gamba S, Perna A, Gaspari F, Remuzzi G, Ruggenenti P. A multidrug, antiproteinuric approach to alport syndrome: a ten-year cohort study. Nephron. 2015:130(1):13-20. doi: 10.1159/000381480. Epub 2015 Apr 21
[PubMed PMID: 25895746]
[50]
Gross O, Tönshoff B, Weber LT, Pape L, Latta K, Fehrenbach H, Lange-Sperandio B, Zappel H, Hoyer P, Staude H, König S, John U, Gellermann J, Hoppe B, Galiano M, Hoecker B, Ehren R, Lerch C, Kashtan CE, Harden M, Boeckhaus J, Friede T, German Pediatric Nephrology (GPN) Study Group and EARLY PRO-TECT Alport Investigators. A multicenter, randomized, placebo-controlled, double-blind phase 3 trial with open-arm comparison indicates safety and efficacy of nephroprotective therapy with ramipril in children with Alport's syndrome. Kidney international. 2020 Jun:97(6):1275-1286. doi: 10.1016/j.kint.2019.12.015. Epub 2020 Jan 17
[PubMed PMID: 32299679]
Level 1 (high-level) evidence
[51]
Nicklason E, Mack H, Beltz J, Jacob J, Farahani M, Colville D, Savige J. Corneal endothelial cell abnormalities in X-linked Alport syndrome. Ophthalmic genetics. 2020 Feb:41(1):13-19. doi: 10.1080/13816810.2019.1709126. Epub 2020 Mar 11
[PubMed PMID: 32159412]
[52]
Antignac C, Knebelmann B, Drouot L, Gros F, Deschênes G, Hors-Cayla MC, Zhou J, Tryggvason K, Grünfeld JP, Broyer M. Deletions in the COL4A5 collagen gene in X-linked Alport syndrome. Characterization of the pathological transcripts in nonrenal cells and correlation with disease expression. The Journal of clinical investigation. 1994 Mar:93(3):1195-207
[PubMed PMID: 8132760]
[53]
Hudson BG, Kalluri R, Gunwar S, Weber M, Ballester F, Hudson JK, Noelken ME, Sarras M, Richardson WR, Saus J. The pathogenesis of Alport syndrome involves type IV collagen molecules containing the alpha 3(IV) chain: evidence from anti-GBM nephritis after renal transplantation. Kidney international. 1992 Jul:42(1):179-87
[PubMed PMID: 1635348]
[54]
Brainwood D, Kashtan C, Gubler MC, Turner AN. Targets of alloantibodies in Alport anti-glomerular basement membrane disease after renal transplantation. Kidney international. 1998 Mar:53(3):762-6
[PubMed PMID: 9507224]
[55]
Kalluri R, van den Heuvel LP, Smeets HJ, Schroder CH, Lemmink HH, Boutaud A, Neilson EG, Hudson BG. A COL4A3 gene mutation and post-transplant anti-alpha 3(IV) collagen alloantibodies in Alport syndrome. Kidney international. 1995 Apr:47(4):1199-204
[PubMed PMID: 7783419]
[56]
Tryggvason K, Zhou J, Hostikka SL, Shows TB. Molecular genetics of Alport syndrome. Kidney international. 1993 Jan:43(1):38-44
[PubMed PMID: 8433568]
[58]
Quérin S, Noël LH, Grünfeld JP, Droz D, Mahieu P, Berger J, Kreis H. Linear glomerular IgG fixation in renal allografts: incidence and significance in Alport's syndrome. Clinical nephrology. 1986 Mar:25(3):134-40
[PubMed PMID: 3514017]
[59]
Kashtan CE, Butkowski RJ, Kleppel MM, First MR, Michael AF. Posttransplant anti-glomerular basement membrane nephritis in related males with Alport syndrome. The Journal of laboratory and clinical medicine. 1990 Oct:116(4):508-15
[PubMed PMID: 2212860]
Level 3 (low-level) evidence
[60]
Kajimoto Y, Endo Y, Terasaki M, Kunugi S, Igarashi T, Mii A, Terasaki Y, Shimizu A. Pathologic glomerular characteristics and glomerular basement membrane alterations in biopsy-proven thin basement membrane nephropathy. Clinical and experimental nephrology. 2019 May:23(5):638-649. doi: 10.1007/s10157-018-01687-1. Epub 2019 Jan 28
[PubMed PMID: 30687875]
[61]
Massella L, Gangemi C, Giannakakis K, Crisafi A, Faraggiana T, Fallerini C, Renieri A, Muda AO, Emma F. Prognostic value of glomerular collagen IV immunofluorescence studies in male patients with X-linked Alport syndrome. Clinical journal of the American Society of Nephrology : CJASN. 2013 May:8(5):749-55. doi: 10.2215/CJN.07510712. Epub 2013 Jan 31
[PubMed PMID: 23371956]
[62]
Jais JP, Knebelmann B, Giatras I, De Marchi M, Rizzoni G, Renieri A, Weber M, Gross O, Netzer KO, Flinter F, Pirson Y, Dahan K, Wieslander J, Persson U, Tryggvason K, Martin P, Hertz JM, Schröder C, Sanak M, Carvalho MF, Saus J, Antignac C, Smeets H, Gubler MC. X-linked Alport syndrome: natural history and genotype-phenotype correlations in girls and women belonging to 195 families: a "European Community Alport Syndrome Concerted Action" study. Journal of the American Society of Nephrology : JASN. 2003 Oct:14(10):2603-10
[PubMed PMID: 14514738]
[63]
Rheault MN. Women and Alport syndrome. Pediatric nephrology (Berlin, Germany). 2012 Jan:27(1):41-6. doi: 10.1007/s00467-011-1836-7. Epub 2011 Mar 5
[PubMed PMID: 21380623]
[64]
Fallerini C, Baldassarri M, Trevisson E, Morbidoni V, La Manna A, Lazzarin R, Pasini A, Barbano G, Pinciaroli AR, Garosi G, Frullanti E, Pinto AM, Mencarelli MA, Mari F, Renieri A, Ariani F. Alport syndrome: impact of digenic inheritance in patients management. Clinical genetics. 2017 Jul:92(1):34-44. doi: 10.1111/cge.12919. Epub 2017 Feb 22
[PubMed PMID: 27859054]