Introduction
The larynx consists of an intricate array of muscles, ligaments, and cartilaginous structures that provide several vital functions. These vary from the protection of the airway during swallowing to the production of voice. Thhis activity will discuss the anatomy and function of these structures and how they interact with one another. Furthermore, it will explore the significant clinical implications in regards to surgical and anesthetic considerations.[1]
Structure and Function
The larynx has a cartilaginous framework that houses the muscles and ligaments that are primarily and uniquely designed to prevent the aspiration of ingested material into the airway. These same structures are involved in the production of voice as airflow from the lungs passes through the vocal folds (housed in the larynx) and creates various frequencies of sound that can ultimately produce a person's unique voiceprint. The larynx subdivides into three parts: the supraglottis (the epiglottis and false or ventricular folds), the glottis (the true vocal folds) and the subglottis. Any alteration in the function of the larynx can affect breathing, swallowing, or voice.
Embryology
As mentioned earlier, the larynx is a multifaceted assembly made up of components that systematically work together to provide vital physiologic functions. The developing larynx arises from branchial structures around the fourth week of gestation. Arising from both the endoderm and mesoderm, several pharyngeal arches give rise to the larynx and its associated structures. More specifically these pharyngeal arches are the third (develops into cranial nerve IX and greater horn of hyoid), fourth (superior laryngeal nerve, thyroid cartilage, cuneiform cartilage, cricopharyngeus muscle, and cricothyroid muscle) and sixth (recurrent laryngeal nerve, cricoid cartilage, arytenoid cartilages, corniculate cartilages, intrinsic laryngeal muscles).
The larynx develops from an outgrowth opening around the fourth week of gestation, termed the laryngotracheal groove. As this groove continues to elongate and progress, the epiglottis and laryngeal inlet begin to appear. Eventually, a septum develops and begins to fuse around the twenty-fifth day of gestation which separates the laryngeal inlet into two distinct compartments, this is the esophagotracheal septum or laryngotracheal septum and arises from the fusion of the tracheoesophageal fold.
The final arrangement following this separation results in a tubular structure (the esophagus) that lies posteriorly, while another tubular structure (the developing respiratory tract) sits anteriorly. Subsequently, there is an additional outpouching, known as the laryngotracheal diverticulum, from the anterior tubular structure which becomes the future larynx, trachea, and lungs.[2][3][4]
Blood Supply and Lymphatics
The larynx is a well-vascularized tubular structure that receives its blood supply from several major vessels. On either side, the larynx receives vascular supply from the right and left common carotid arteries which bifurcate into the internal and external carotid arteries. The first branch from the external carotid artery is the superior thyroid artery. This artery further divides into the superior laryngeal artery, which pierces the thyrohyoid membrane to supply the intrinsic muscles of the larynx, and the cricothyroid branch that supplies the cricothyroid muscle. Venous drainage is accomplished ultimately by the internal jugular vein which divides into the corresponding vessels of the superior thyroid vein as well as both the superior and inferior laryngeal veins. Lymphatic drainage occurs through a combination of deep cervical lymph nodes such as the anterior cervical and lateral jugular lymph nodes which terminate at the right lymphatic duct and thoracic duct.[4]
Nerves
The laryngeal muscles receive innervation from two nerves, the external branch of the superior laryngeal nerve and the recurrent laryngeal nerve. These nerves both arise from the vagus nerve (cranial nerve X) and provide both motor and sensory innervation to the intrinsic muscles of the larynx, excluding the interarytenoid muscles. The cricothyroid muscle is innervated solely by the external branch of the superior laryngeal nerve while the recurrent laryngeal provides motor innervates to the other intrinsic laryngeal muscles. The internal branch of superior laryngeal nerve supplies sensory to the mucosa of the larynx. The nucleus ambiguous within the brain stem provides the motoneurons to the intrinsic laryngeal muscles while the hypoglossal nucleus provides the motoneurons to extrinsic muscles.[5] The recurrent laryngeal branch of the vagus nerve descends in the neck and on the left side loops around the aorta before ascending and entering the cricothyroid membrane to innervate the muscles. On the right the nerve loops around the subclavian and ascends to enter the larynx. Non-recurrent anatomic variants on the right do exist.
Muscles
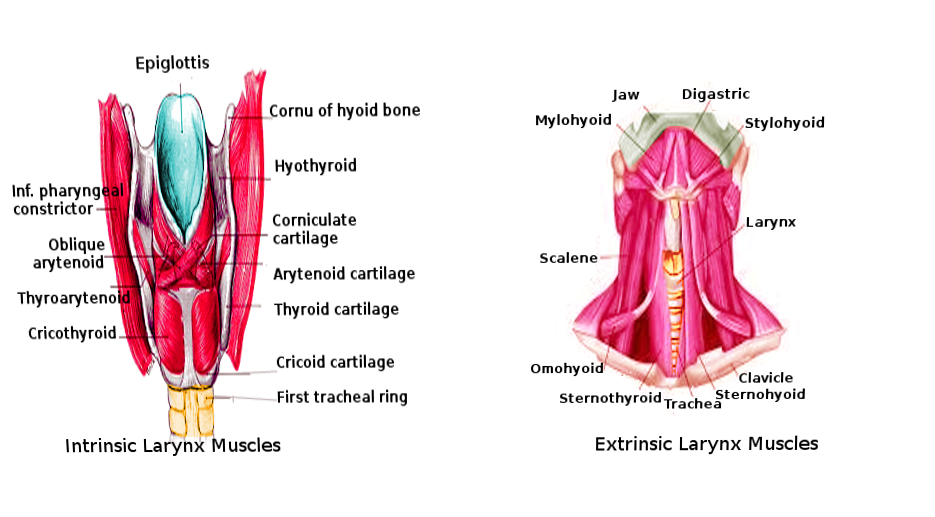
Comprising the larynx are both intrinsic and extrinsic muscles that play a part in the manipulation of air movement, swallowing, and the production of voice. These muscles participate in the orchestrated control of vocal cord abduction, adduction, and lengthening. The four paired, and one unpaired intrinsic muscles are those which are localized to the larynx while extrinsic muscles involve those which attach to both the larynx and other proximity structures. These intrinsic muscles of the larynx include the paired thyroarytenoid, lateral cricoarytenoid, posterior cricoarytenoid, and cricothyroid muscles and the unpaired interarytenoid muscle — all the intrinsic muscles except the posterior cricoarytenoid act to adduct the vocal folds. The action of abduction is a result of posterior cricoarytenoid activation. The cricothyroid muscle is unique in that it elongates the vocal cords as well, which creates tension on the vocal cords and assists in high pitch phonation. The extrinsic muscles of the larynx such as the thyrohyoid (which raises the thyroid cartilage) and the sternothyroid muscle (which lowers the thyroid cartilage) function to manipulate the position of the larynx during swallowing or pitch control during phonation.[5][6][7]
Physiologic Variants
As with any anatomical structure of the body, there exists the potential for variants. Such variants can arise as congenital malformations leading to impaired respiratory function in the neonate. One such anomaly involves the presence of laryngeal clefts in the newborn, which can pose a risk for aspiration and respiratory distress. Such clefts are a result of a failed separation of the previously mentioned laryngotracheal septum leading to a lack of separation between the larynx/trachea and the esophagus. Other considerations occur when there is incomplete canalization of the developing larynx leading to various degrees of obstruction of the airway, which should be a consideration in an infant that develops respiratory distress.
Variations also exist anatomically with the neurovascular arrangement of the laryngeal structures. Cases of a nonrecurrent inferior laryngeal nerve have been recorded on the right, representing a rare anatomical anomaly thought to be a result of the fourth branchial arch involuting instead of persisting as the subclavian artery. The nonrecurrent laryngeal nerve then branches directly off the cervical vagus. The positional relationship between the recurrent laryngeal nerve and the inferior thyroid artery can vary in position with the recurrent laryngeal nerve sitting either posterior, anterior, or between branches of the inferior thyroid artery. The branching pattern of the recurrent laryngeal nerve can also vary from two to many branches within the musculature and cartilaginous framework. In general, all of these varieties are important when considering thyroid or other laryngotracheal surgical operations.[8][3]
Surgical Considerations
Iatrogenic causes of injury to the vagus nerve and its branches can lead to a unilateral paresis or paralysis of the vocal fold. Recurrent laryngeal nerve damage can be a complication in operations such as thyroid surgery where unilateral damage leads to hoarseness or swallowing difficulties. Damage to the external branch of the superior laryngeal nerve can lead to an inability to increase pitch as the cricothyroid is unable to lengthen the vocal folds. Bilateral damage to the nerves, while extremely uncommon, can have severe sequelae of respiratory distress (secondary to failure of abduction and resultant midline position of the vocal folds) and occasionally can lead to a tracheostomy.[7][8][5][9]
During endotracheal intubation, the endotracheal tube passes through the vocal folds, and the end ultimately rests in the subglottic larynx. Ischemic damage from the pressure of the endotracheal tube through weaknesses in the cartilaginous framework can occur and ultimately cause sequela to the recurrent laryngeal nerve, resulting in apraxia that usually resolves with time. Further insults of the passage of the endotracheal tube through the larynx involve damage or hemorrhage to the vocal folds and possible glottic and subglottic inflammation and subsequent scar formation. This scar formation may obstruct the airway and lead to distress in the long-term.
Clinical Significance
There are a variety of important implications when considering the surgical, anesthetic, and clinical significance of the larynx. Clinical evaluation of the larynx in any patient with voice, swallowing, or airway symptoms is vital to determine functional or anatomic anomalies that may be a cause. Understanding the anatomy and function of the organ and its impact on the complex orchestration of swallowing and voicing will better equip the clinician to educate and treat patients with disorders of the upper aerodigestive tract.