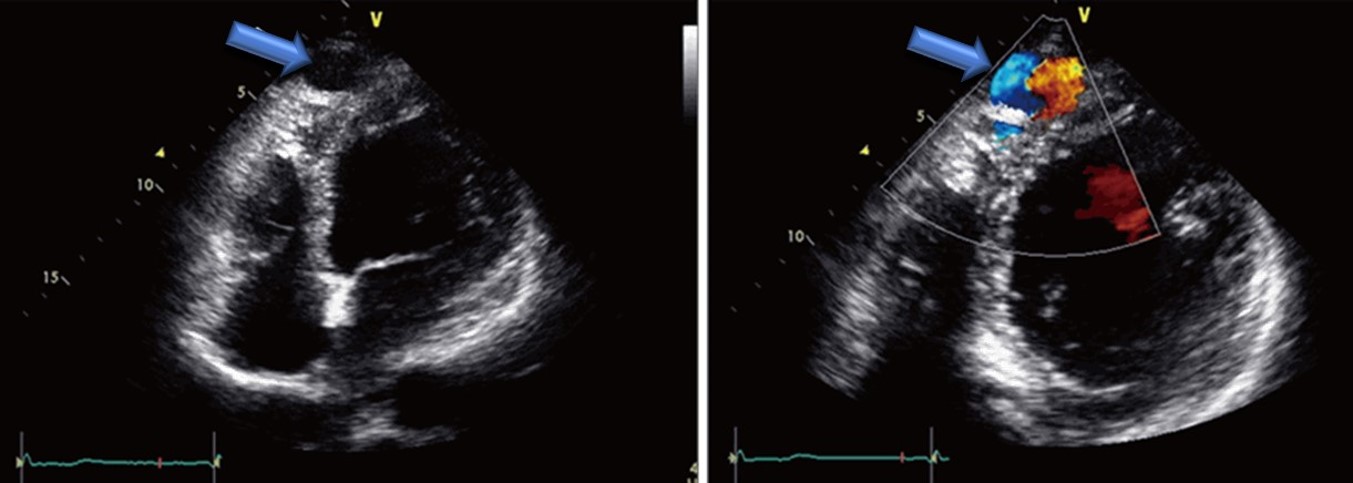
[1]
Sağlam H, Koçoğullari CU, Kaya E, Emmiler M. Congenital coronary artery fistula as a cause of angina pectoris. Turk Kardiyoloji Dernegi arsivi : Turk Kardiyoloji Derneginin yayin organidir. 2008 Dec:36(8):552-4
[PubMed PMID: 19223723]
[2]
Minhas AM, Ul Haq E, Awan AA, Khan AA, Qureshi G, Balakrishna P. Coronary-Cameral Fistula Connecting the Left Anterior Descending Artery and the First Obtuse Marginal Artery to the Left Ventricle: A Rare Finding. Case reports in cardiology. 2017:2017():8071281. doi: 10.1155/2017/8071281. Epub 2017 Jan 17
[PubMed PMID: 28194284]
Level 3 (low-level) evidence
[3]
Sharma UM, Aslam AF, Tak T. Diagnosis of coronary artery fistulas: clinical aspects and brief review of the literature. The International journal of angiology : official publication of the International College of Angiology, Inc. 2013 Sep:22(3):189-92. doi: 10.1055/s-0033-1349166. Epub
[PubMed PMID: 24436610]
[4]
Abdelmoneim SS, Mookadam F, Moustafa SE, Holmes DR. Coronary artery fistula with anomalous coronary artery origin: a case report. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2007 Mar:20(3):333.e1-4
[PubMed PMID: 17336762]
Level 3 (low-level) evidence
[5]
Vitarelli A, De Curtis G, Conde Y, Colantonio M, Di Benedetto G, Pecce P, De Nardo L, Squillaci E. Assessment of congenital coronary artery fistulas by transesophageal color Doppler echocardiography. The American journal of medicine. 2002 Aug 1:113(2):127-33
[PubMed PMID: 12133751]
[6]
Lim JJ, Jung JI, Lee BY, Lee HG. Prevalence and types of coronary artery fistulas detected with coronary CT angiography. AJR. American journal of roentgenology. 2014 Sep:203(3):W237-43. doi: 10.2214/AJR.13.11613. Epub
[PubMed PMID: 25148179]
[7]
Zhou K, Kong L, Wang Y, Li S, Song L, Wang Z, Wu W, Chen J, Wang Y, Jin Z. Coronary artery fistula in adults: evaluation with dual-source CT coronary angiography. The British journal of radiology. 2015 May:88(1049):20140754. doi: 10.1259/bjr.20140754. Epub 2015 Mar 18
[PubMed PMID: 25784320]
[8]
Chen ML, Lo HS, Su HY, Chao IM. Coronary artery fistula: assessment with multidetector computed tomography and stress myocardial single photon emission computed tomography. Clinical nuclear medicine. 2009 Feb:34(2):96-8. doi: 10.1097/RLU.0b013e318192c497. Epub
[PubMed PMID: 19352262]
[9]
Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, del Nido P, Fasules JW, Graham TP Jr, Hijazi ZM, Hunt SA, King ME, Landzberg MJ, Miner PD, Radford MJ, Walsh EP, Webb GD. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Circulation. 2008 Dec 2:118(23):e714-833. doi: 10.1161/CIRCULATIONAHA.108.190690. Epub 2008 Nov 7
[PubMed PMID: 18997169]
Level 1 (high-level) evidence
[10]
McMahon CJ, Nihill MR, Kovalchin JP, Mullins CE, Grifka RG. Coronary artery fistula. Management and intermediate-term outcome after transcatheter coil occlusion. Texas Heart Institute journal. 2001:28(1):21-5
[PubMed PMID: 11330735]
[11]
Armsby LR, Keane JF, Sherwood MC, Forbess JM, Perry SB, Lock JE. Management of coronary artery fistulae. Patient selection and results of transcatheter closure. Journal of the American College of Cardiology. 2002 Mar 20:39(6):1026-32
[PubMed PMID: 11897446]
[12]
Edwards FH, Engelman RM, Houck P, Shahian DM, Bridges CR, Society of Thoracic Surgeons. The Society of Thoracic Surgeons Practice Guideline Series: Antibiotic Prophylaxis in Cardiac Surgery, Part I: Duration. The Annals of thoracic surgery. 2006 Jan:81(1):397-404
[PubMed PMID: 16368422]
Level 1 (high-level) evidence
[13]
Engelman R, Shahian D, Shemin R, Guy TS, Bratzler D, Edwards F, Jacobs M, Fernando H, Bridges C, Workforce on Evidence-Based Medicine, Society of Thoracic Surgeons. The Society of Thoracic Surgeons practice guideline series: Antibiotic prophylaxis in cardiac surgery, part II: Antibiotic choice. The Annals of thoracic surgery. 2007 Apr:83(4):1569-76
[PubMed PMID: 17383396]
Level 1 (high-level) evidence