Continuing Education Activity
Sepsis secondary to peritonitis can have life-threatening ramifications. The abdominal compartment can become a large nidus for pathogens resistant to standard therapy. On top of that, the abdominal compartment serves as a conduit to other vital organs, which can hasten the spread of resistant pathogens. This activity explains when septic peritonitis should be considered on a differential diagnosis, articulates how to properly evaluate for this condition, and highlights the role of the interprofessional team in caring for patients with this condition.
Objectives:
Identify the common mechanisms responsible for the development of septic peritonitis.
Review risk factors for developing septic peritonitis.
Identify pertinent evaluation findings associated with the development of septic peritonitis.
Employ interprofessional team strategies for improving care coordination and communication to advance the recognition and appropriate management of septic peritonitis.
Introduction
Sepsis is a proinflammatory and anti-inflammatory response that manifests in the body as a result of the host-pathogen interaction, with excessive inflammation resulting in collateral tissue injury, and the anti-inflammatory response resulting in immunosuppression, leading to increased susceptibility to secondary infection.
The clinical manifestations of sepsis are highly variable, depending on the initial site of infection, the causative organism, the pattern of acute organ dysfunction, the underlying health status of the patient, and the interval before initiation of treatment.
Severe sepsis occurs as a result of both community-acquired and healthcare-associated infections. While pneumonia continues to be the most common cause of sepsis, both community-associated and in the healthcare setting, intraabdominal and urinary tract infections are important conduits to the pathological systemic response.[1] Staphylococcus aureus and S. pneumoniae are the most common gram-positive pathogens, and Escherichia coli, Klebsiella species, and Pseudomonas aeruginosa standout among the gram-negative microorganisms.[2]
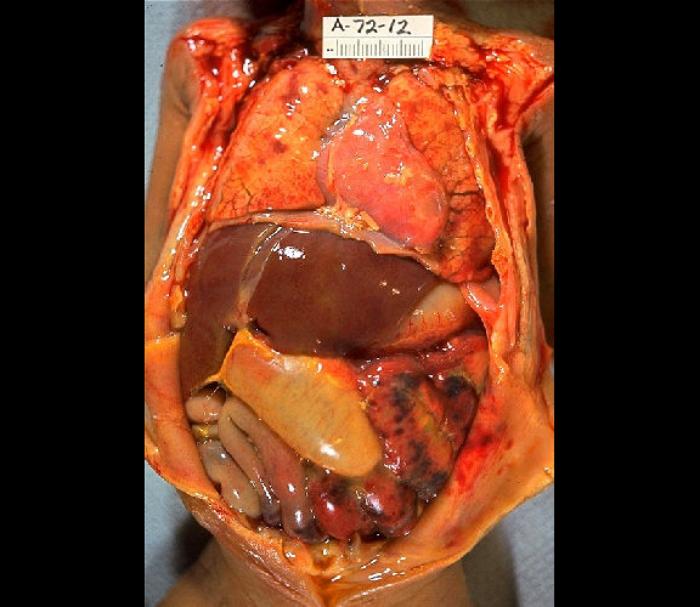
When an infection in the intra-abdominal cavity has a primary focus, with identifiable localization, it is called an abscess; when the nidus of the inflammatory response occurs in the serous membrane lining the cavity, it is called peritonitis. Both can potentially lead to a systemic inflammatory/anti-inflammatory response known as peritonitis-induced sepsis. This review will focus on the fundamental tenets of diagnosing and treating the underlying infection and the complications associated with sepsis. See Image. Severe Septic Peritonitis.
Etiology
The clinically significant growth of pathogenic microorganisms into the sterile abdominal cavity is most often accomplished by violation (most commonly perforation) of the gastrointestinal (GI) viscera anywhere along the track, which can be precipitated by a chronic disease process and make tissue friable and more susceptible to rupture, or an acute onset disease (e.g., cholecystitis, pancreatitis). Mechanical violations of the abdominal wall are also responsible for a significant proportion of infections, including but not limited to trauma, surgery, and seeding from dialysis catheters. To a lesser degree, an infection can manifest as a result of hematogenous spread in immunocompromised patients and spontaneous bacterial peritonitis in individuals with ascitic fluid collection resultant from cirrhosis, congestive heart failure (CHF), and nephrotic disease.
Epidemiology
Several papers dealing with the regional incidence of peritonitis induced sepsis in a particular population have reported inconclusive data which cannot be extrapolated to generalized observations. However, when dealing specifically with a sub-group of peritonitis, the reported incidence of spontaneous bacterial peritonitis (SBP) in a patient with ascites is estimated to be between 7% to 30% per year.[3]
Pathophysiology
Gram-negative and/or anaerobic organisms are the usual suspects responsible for infection when perforation is the mechanism of inoculation. Gut flora (E. coli, K. pneumoniae, etc.) then release endotoxins that are responsible for the overreactive inflammatory cascade that causes sepsis.
Decreased fibrinolysis mediated through increased plasminogen activator inhibitor activity which results in an increase of fibrin exudates facilitates large bacterial loads being sequestered in the fibrin matrix. The host's logic behind this process is to retard the spread of bacteria beyond the local nidus, but this action also results in creating a physical barrier to advancing host defense mechanisms, thereby in some cases, prolonging the initial infection and/or abscess formation. See Image. Gross Pathology of Neonatal Necrotizing Enterocolitis.
History and Physical
A good history could give insight into the origins of inoculation. A recent history of abdominal surgery, chronic inflammatory disease, end-stage renal disease, and chronic use of immunosuppressive medication are all important in triggering an appropriate investigation and workup.
Concerning signs present in a high percentage of individuals with diagnosed peritonitis include vague constitutional symptoms such as fever chills, abdominal pain +/- discomfort, diarrhea, and ileus. Important to note, approximately 30% of individuals with SBP will be asymptomatic on presentation.
During the physical exam, pertinent findings include fever and abdominal tenderness to palpation which usually is diffuse with wall rigidity in more septic presentations.
It is important to conduct a thorough exam as certain thoracic or pelvic pathologies can mimic peritoneal irritation (empyema causing diaphragmatic irritation and cystitis/pyelonephritis causing peritoneum adjacent pain).
Evaluation
Patients will present with a variable amount of clinical manifestation of the underlying disease process, ranging from insidious mild limited disease to an acute fulminant systemic process. As such, the diagnosis is usually clinical with some laboratory findings to aid in treatment regimens. The following are pertinent laboratory findings important to mortality and morbidity:
- Leukocytosis in the CBC, evidence of renal and/or hepatic dysfunction (via CMP +/- UA, denoting possible MOF or pre-existing comorbidities)
- Positive blood cultures, and
- Peritoneal fluid analysis
Fluid analysis most importantly for the Dx of SBP, with the most sensitive indicator being a neutrophil count greater than 250 cells/microliter. The fluid should also be analyzed for glucose, protein, LDH, cell count, Gram stain, and aerobic and anaerobic cultures. The SAAG (serum-ascites albumin gradient) is used to determine the composition of the ascitic fluid (transudative vs. exudative), with the DDx depending on the gradient, a gradient greater than 1.1 g/dL is indicative of a transudative fluid related to increased hydrostatic pressures, such as portal-HTN and/or thrombosis, CHF, and Budd–Chiari syndrome to name a few. A low SAAG (less than 1.1 g/dL) indicating fluid associated with an increase in portal pressures, e.g., infections (tuberculosis, SBP), neoplasms, nephrotic disease, and other inflammatory processes such as pancreatitis and serositis.[4]
The severity of the illness is managed with careful consideration of sepsis protocols, including monitoring parameters reflective of end-organ tissue perfusion mean arterial pressure, lactate level, evidence of end-organ damage such as encephalopathy, renal dysfunction, and evaluation for the necessity of supportive care measures like ventilatory or pressor support.
Treatment / Management
The primary focus of management is the identification and targeted treatment of offending agent(s) via antibiotics and/or surgical intervention. The non-operative measure includes broad-spectrum antibiotic administration with appropriate stewardship, tailoring the regimen to accomplish increased efficacy targeting the identified microorganisms. Ultrasound/CT-guided abscess drainage, percutaneous/endoscopic stent placement and important non-surgical interventions.[5] Supplemental therapies are focused on diminishing the effects of toxin release, end-organ damage, and host-mediated inflammatory response pathognomonic for sepsis.
Differential Diagnosis
Because of the broad range of sometimes vague constitutional symptoms, the differential for the observed symptomatology can be wide. Anatomical location is important in considering the other possible disease processes that have to be ruled out. Pathology in all four surrounding surfaces have to be considered including:
- Diaphragmatic irritation (elicited by thoracic conditions, e.g., empyema, inferiorly located pleural disease)
- Pelvic pathology (e.g., cystitis, urinary obstruction)
- Retroperitoneal diseases (e.g., pyelonephritis, hematoma)
- Abdominal wall issues (e.g., rectus infection/hematoma).
Also, the following can be causes of inflammation outside of the abdominal cavity:
- Chemical irritants (e.g., bile, gastric juices, blood).
- Systemic inflammatory disease with intra-abdominal manifestations (e.g., SLE, Crohn, allergic vasculitis).
- In addition, some vascular abnormalities can cause widespread inflammatory responses including mesenteric ischemia/thrombosis, ischemic colitis.
Enhancing Healthcare Team Outcomes
A well functioning efficient interprofessional team is necessary to execute the appropriate clinical measures needed to treat patients with sepsis because time management is critical in predicting the overall outcomes. A well-established consensus committee of 55 international experts, representing 25 international organizations, formulated the Surviving Sepsis Campaign (SSC) guidelines with the most recent iteration developed in 2016. In the guidelines, recommendations are made concerning the hospital-derived analytical processes that should be implemented to assess the effectiveness of each institution's sepsis protocol. The proposals include target metrics to monitor goal attainment, data collection, and ongoing feedback criticizing the effectiveness of the program. These recommendations are made based on the meta-analysis of 50 observational studies, illustrating that performance improvement programs had a direct correlation with a significant increase in compliance with the study's guidelines, thus a reduction in mortality. It is not enough to simply mandate compliance with an established sepsis protocol; there must be a recognized method by which adherence, efficiency of execution, and overall outcomes can be analyzed.[6]