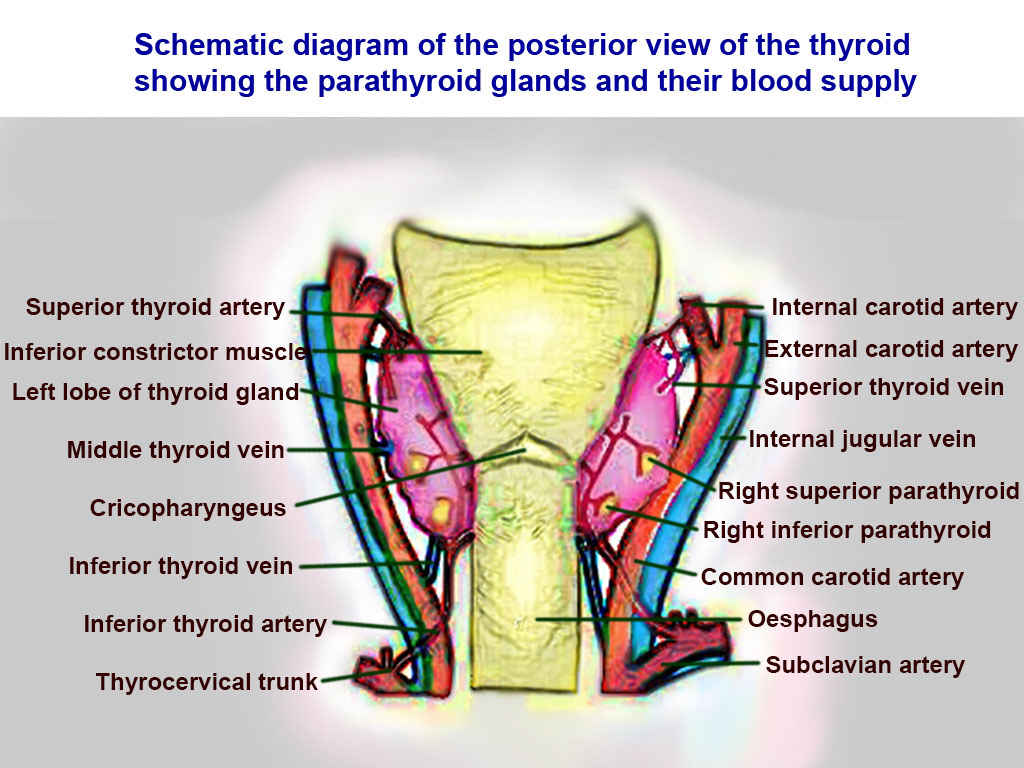
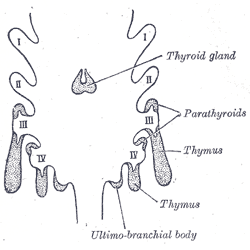
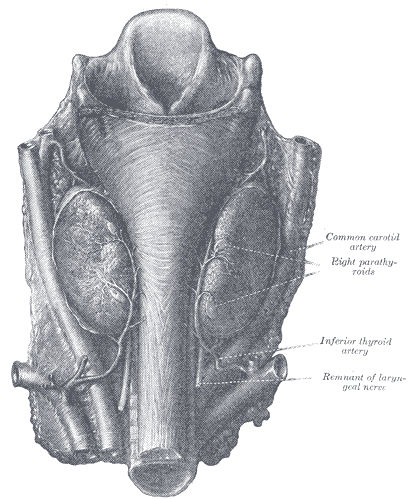
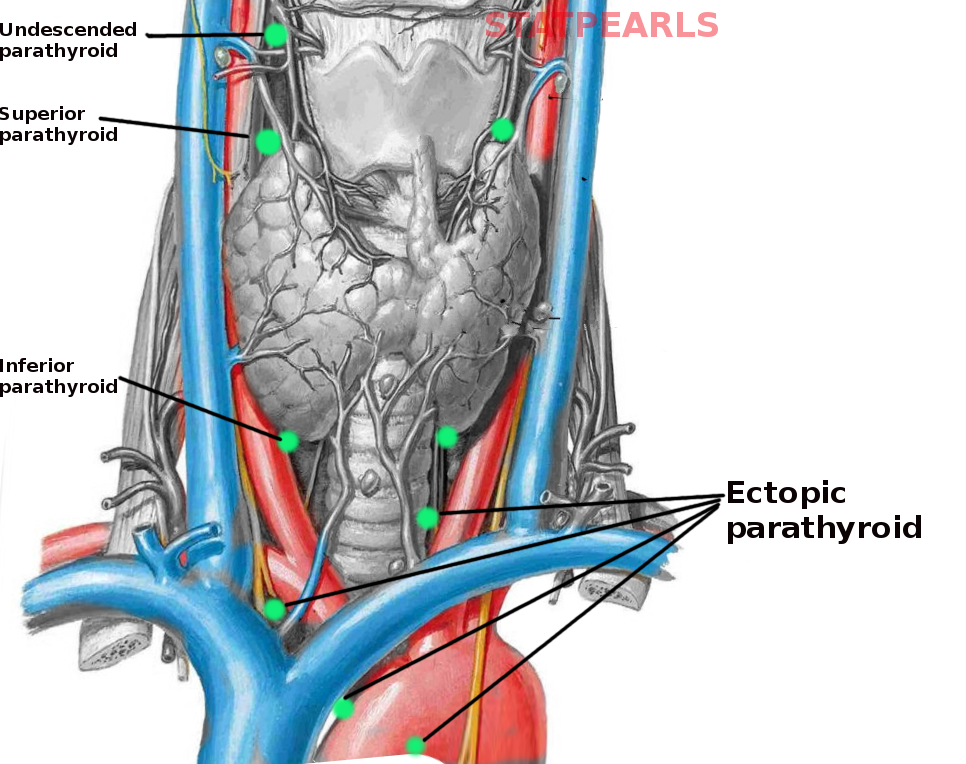
[2]
Georgakopoulos B, Al Khalili Y. Anatomy, Head and Neck, Parathyroid, Ectopic Glands. StatPearls. 2023 Jan:():
[PubMed PMID: 31082016]
[6]
Tanaka Y, Funahashi H, Imai T, Seo H, Tominaga Y, Takagi H. Oxyphil cell function in secondary parathyroid hyperplasia. Nephron. 1996:73(4):580-6
[PubMed PMID: 8856255]
[11]
Blanco I, Carril JM, Banzo I, Quirce R, Gutierrez C, Uriarte I, Montero A, Vallina NK. Double-phase Tc-99m sestamibi scintigraphy in the preoperative location of lesions causing hyperparathyroidism. Clinical nuclear medicine. 1998 May:23(5):291-7
[PubMed PMID: 9596153]
[12]
Bann DV, Zacharia T, Goldenberg D, Goyal N. Parathyroid localization using 4D-computed tomography. Ear, nose, & throat journal. 2015 Apr-May:94(4-5):E55-7
[PubMed PMID: 25923289]
[13]
Brown SJ, Lee JC, Christie J, Maher R, Sidhu SB, Sywak MS, Delbridge LW. Four-dimensional computed tomography for parathyroid localization: a new imaging modality. ANZ journal of surgery. 2015 Jun:85(6):483-7. doi: 10.1111/ans.12571. Epub 2014 Mar 27
[PubMed PMID: 24674300]
[20]
Lindblom P, Westerdahl J, Bergenfelz A. Low parathyroid hormone levels after thyroid surgery: a feasible predictor of hypocalcemia. Surgery. 2002 May:131(5):515-20
[PubMed PMID: 12019404]
[24]
Noussios G, Anagnostis P, Natsis K. Ectopic parathyroid glands and their anatomical, clinical and surgical implications. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association. 2012 Nov:120(10):604-10. doi: 10.1055/s-0032-1327628. Epub 2012 Nov 22
[PubMed PMID: 23174995]
[25]
Phitayakorn R, McHenry CR. Incidence and location of ectopic abnormal parathyroid glands. American journal of surgery. 2006 Mar:191(3):418-23
[PubMed PMID: 16490559]
[26]
Callender GG, Grubbs EG, Vu T, Hofstetter WL, Fleming JB, Woodburn KL, Lee JE, Evans DB, Perrier ND. The fallen one: the inferior parathyroid gland that descends into the mediastinum. Journal of the American College of Surgeons. 2009 May:208(5):887-93; discussion 893-5. doi: 10.1016/j.jamcollsurg.2009.01.032. Epub
[PubMed PMID: 19476855]
[27]
Patrinos A, Zarokosta M, Piperos T, Tsiaoussis J, Noussios G, Mariolis-Sapsakos T. An anatomic aberration and a surgical challenge: Mediastinal parathyroid adenoma anterior the pericardium. A case report. International journal of surgery case reports. 2019:58():153-156. doi: 10.1016/j.ijscr.2019.04.005. Epub 2019 Apr 6
[PubMed PMID: 31048210]
Level 3 (low-level) evidence
[28]
Miura D. Ectopic parathyroid tumor in the sternohyoid muscles: supernumerary gland in a patient with MEN type 1. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2005 Aug:20(8):1478-9
[PubMed PMID: 16007345]
[29]
Liechty RD, Weil R 3rd. Parathyroid anatomy in hyperplasia. Archives of surgery (Chicago, Ill. : 1960). 1992 Jul:127(7):813-5; discussion 815-6
[PubMed PMID: 1524481]
[31]
Kuo EJ, Al-Alusi MA, Du L, Shieh A, Livhits MJ, Leung AM, Yeh MW. Surgery for Primary Hyperparathyroidism: Adherence to Consensus Guidelines in an Academic Health System. Annals of surgery. 2019 Jan:269(1):158-162. doi: 10.1097/SLA.0000000000002474. Epub
[PubMed PMID: 28806302]
Level 3 (low-level) evidence
[33]
Dobrinja C, Santandrea G, Giacca M, Stenner E, Ruscio M, de Manzini N. Effectiveness of Intraoperative Parathyroid Monitoring (ioPTH) in predicting a multiglandular or malignant parathyroid disease. International journal of surgery (London, England). 2017 May:41 Suppl 1():S26-S33. doi: 10.1016/j.ijsu.2017.02.063. Epub
[PubMed PMID: 28506410]
[34]
Yeung M. Parathyroidectomy Without the Utilisation of iPTH: The Gold Standard is Still a Good Operation-How Understanding the Anatomy and a Simple US Can Help. World journal of surgery. 2020 Feb:44(2):622-624. doi: 10.1007/s00268-019-05217-2. Epub
[PubMed PMID: 31602517]
Level 3 (low-level) evidence
[35]
Joliat GR, Guarnero V, Demartines N, Schweizer V, Matter M. Recurrent laryngeal nerve injury after thyroid and parathyroid surgery: Incidence and postoperative evolution assessment. Medicine. 2017 Apr:96(17):e6674. doi: 10.1097/MD.0000000000006674. Epub
[PubMed PMID: 28445266]