Treatment / Management
Surgical resection is the main treatment modality for localized non-metastatic stage Cca at any age with acceptable performance status and optimized comorbidities. Endoscopic resection (ER) is reserved for selected favorable-risk and early-stage colon carcinomas found in a polyp (cT0-1). Neoadjuvant therapy is not standard of care for Cca and reserved for advanced disease surgical conversion intend. Adjuvant therapy is recommended for all Cca stage III (node-positive) and individualized by stage II with high-risk features. Surgery in conjunction with peri-chemotherapy may provide a curative option on oligo-metastatic lung and liver disease. [17][18][6]Palliative systemic chemotherapy is offered to non-surgical candidates with unresectable locally advanced disease or high metastatic burden to improved quality of life and prolongs life expectancy. Individualized local-recurrent disease patients may achieve cure with further multimodality therapy. Rectal cancer treatment modalities are discussed in a different StatPearls chapter.
Endoscopic Resection
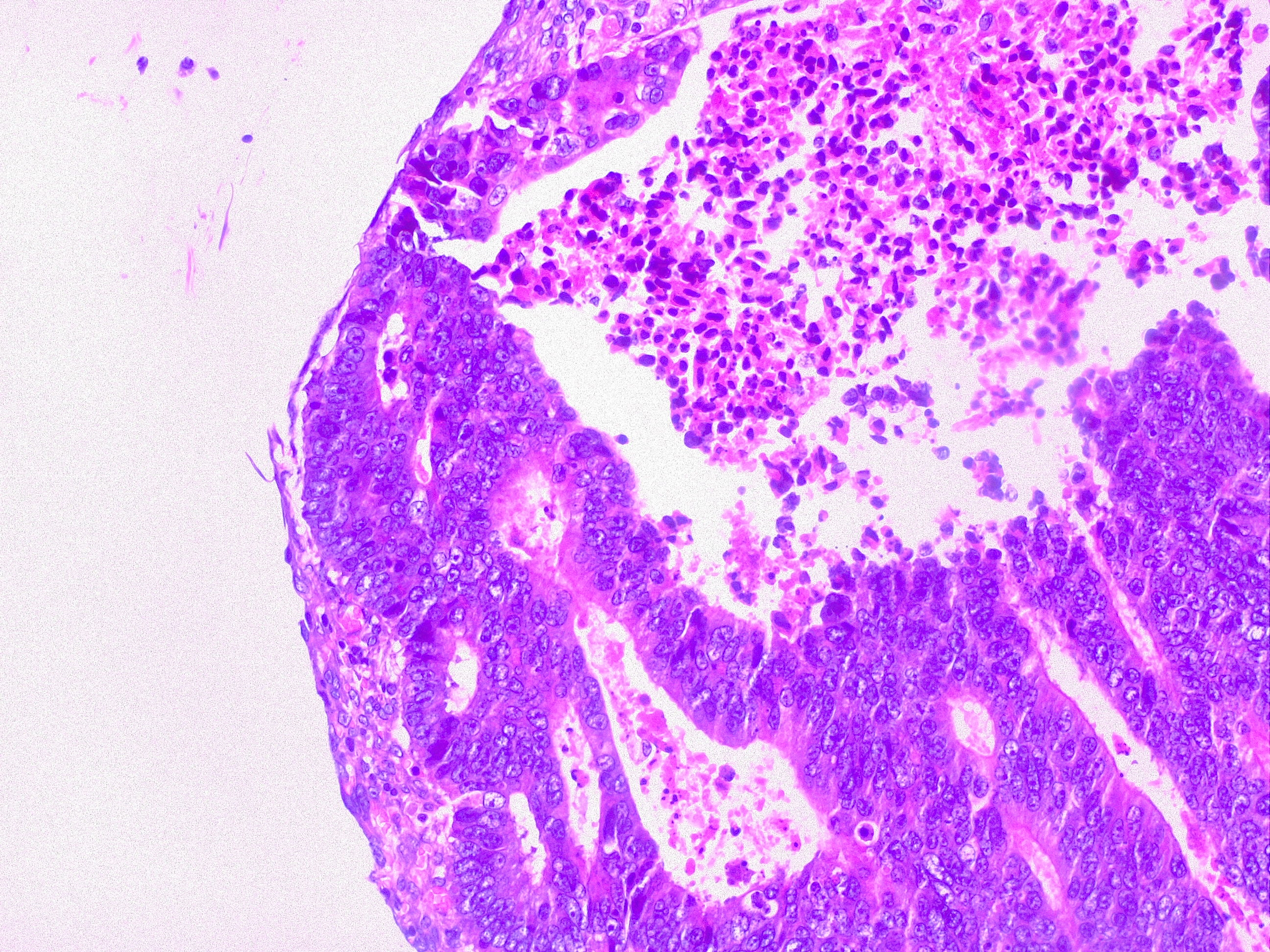
Local excisions by endoscopy should achieve complete tumor resection and adjacent tissue in one block. ER is reserved for patients agreeable to a close, aggressive surveillance afterward, a surgical resection for high-risk disease and/or non-surgical candidates. ER procedures are to be an offer by an experienced physician at the center of excellence. Invasion of muscularis propia (T2), poor histological grade, LVI, PNI, at the stalk or flat/depressed sessile polyp are considered ER, Cca, high-risk features and further need surgical resection. Cca polyp techniques include endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) with high success rate 90.3% (95% CI 88.2% to 92.5%) and a low recurrence 13.8% (95% CI 12.9% to 14.7%), being an invasive cancer in 0.3% (95% CI 0.1% to 0.4%) on favorable risk patients. Major complications are perforation and bleeding with 6.5% and 1.5% respectively and extremely low-mortality 0.8% (95% CI 0.01% to 0.15%). Regardless of the procedure, all lesions should be tattooed to allow site identification on further required steps.
Surgical Resection
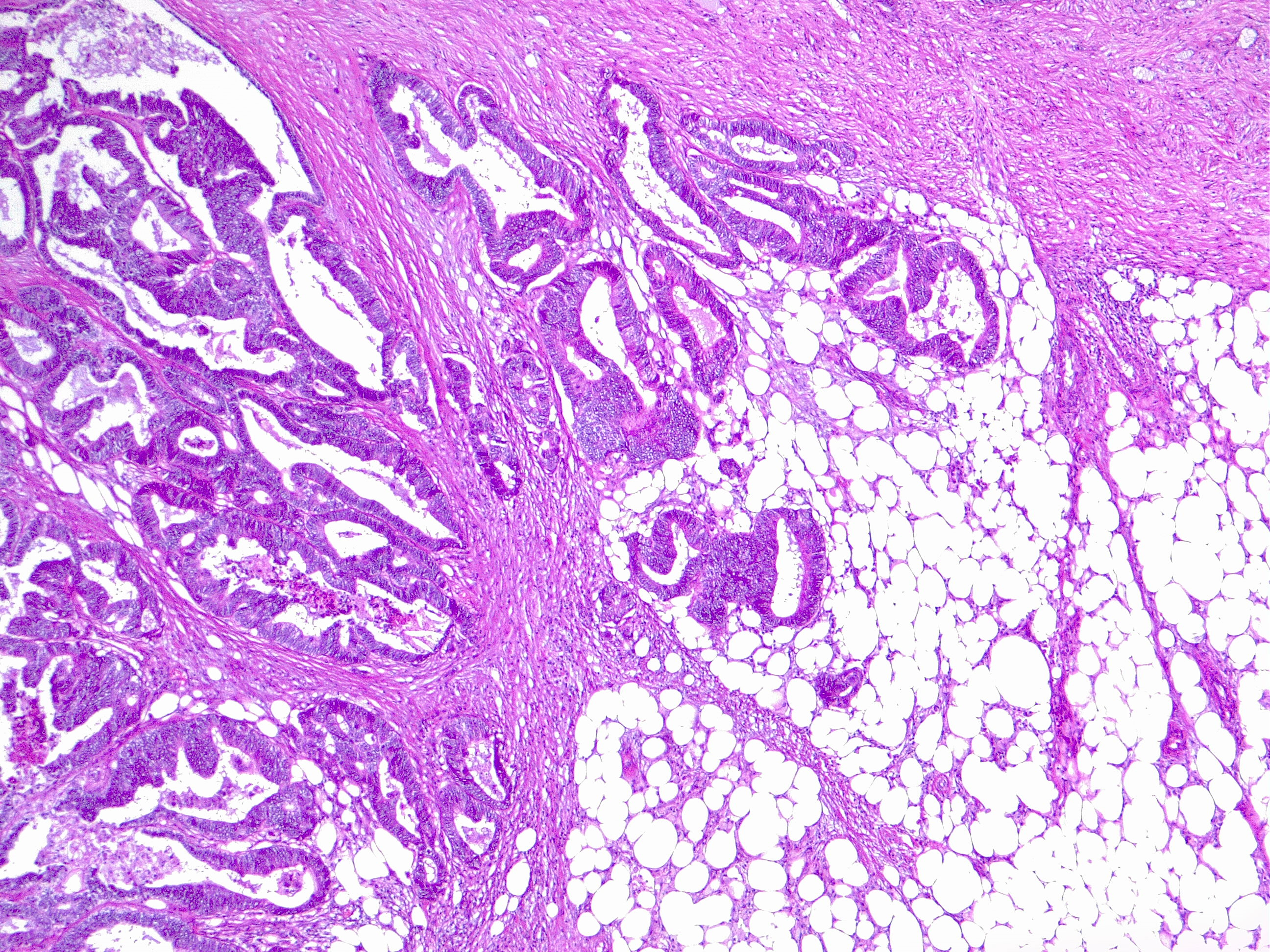
The main goal for invasive Cca is the complete resection of the tumor and potential lymphovascular spread by a goal of a minimal negative proximal and distal margin of 5 cm for colon cancer, and minimal proximal margin of 5 cm and distal of 2 cm for rectal carcinoma. Circumferential/radial margin should be greater than 1 mm. The essentials of oncologic resection involve central vascular ligation of the feeding main artery and complete mesocolic resection of the involved colonic section. Oncologic resection requires removal of 12 or more lymph nodes. The initial step for colectomy is accessing the retroperitoneum and elevating the mesentery of the colon contained within the fascia of Toltd. In laparoscopic procedures, this dissection is started medial to lateral fashion, but in open procedures, this dissection is done lateral to medial way. A secondary aim is the restoration of the bowel continuity, either by one-stage primary anastomosis or two-stage approach with temporary diversion. Conventional open colectomy or laparoscopic colectomy is the preferred surgical modality. High-volume, experienced surgeons should perform the laparoscopic approach. Experience with robotic surgery is under further investigation. Potential palliative surgical procedures for unresectable Cca tumors include resection with primary anastomosis, diverting colostomy, and internal bypass procedures.
Tumors of the appendix, cecum, ascending colon, and hepatic flexure requires right colectomy with ligation of the right colic artery at its origin. The right branch of the middle colic artery and ileocolic artery are also ligated. This procedure is completed with ill -mid-transverse colon anastomosis. Transverse colon cancers require extended right colectomy due to the ease of the procedure when compared to extended left colectomy. Extended right colectomy also involves the ligation of the middle colic artery at its origin in addition to steps involving right colectomy. This procedure is completed with ileac-descending colon anastomosis.
Patients with a distal transverse colon, splenic flexure, and descending colon cancers require left colectomy. This procedure involves the ligation of the inferior mesenteric artery at its origin, as well as ligation of the inferior mesenteric vein at the inferior border of the pancreas. The left branch of the middle colic artery is also ligated. This procedure if completed with transverse colon sigmoid or rectal anastomosis. Patients with sigmoid colon cancers undergo extended left colectomy with colorectal anastomosis.
Patients with synchronous colon cancers (two or more foci) involving the intra-abdominal colon, with distal malignant distal colonic obstruction, with Lynch (HNPCC), and with attenuated FAP should undergo total abdominal colectomy; including cecum, ascending, transverse, descending and sigmoid colons. Therefore this procedure involves ligation of ileocolic, right and middle colic, and inferior mesenteric arteries. This procedure is completed with ileorectal anastomosis.
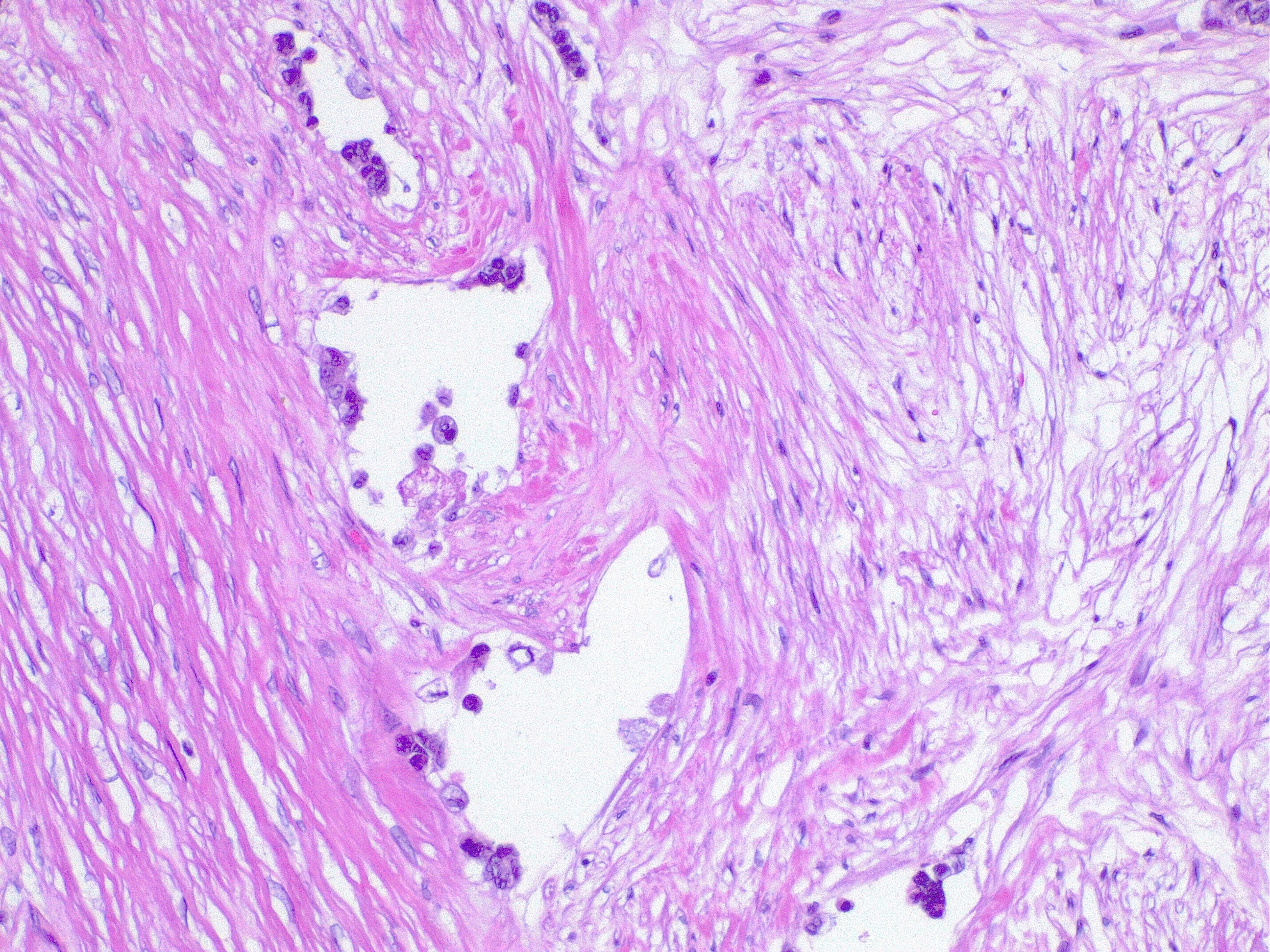
Meta-analysis of randomized clinical trials (including COLOR trial, CLASSIC trial, and COST trial) indicates that laparoscopic-assisted colectomy surgery for Cca provides the same outcomes for 5-year overall survival (OS) (69% versus 68%) and disease-free survival (DFS) (76% versus 75%) as open laparotomy. Reported conversion rates from laparoscopic to open remains at 20% as described in the United States Intergroup Clinical Outcomes of Surgical Therapy (COST) trial. Positive margins have been retrospectively found in 5.3% of Cca resection with a worse OS (hazard ratio [HR] 3.39, 95% CI 2.41 to 4.77). Sentinel lymph node biopsy is not considered a standard of care and consensus guidelines recommend 12 lymph nodes or more with improved 5-year OS at 90% regardless open or laparoscopic approach. Locoregional recurrence can occur up to 12% of Cca resection and is classified into four categories: anastomotic; mesenteric/nodal; retroperitoneal; and peritoneal. Poor prognostic factors include more than one site of recurrence and involvement of the mesentery/nodal basin whereas the ability to obtain an R0 resection was the strongest predictor of outcome, and these patients had a median survival of 66 months.[19]
Surgery alone can be curative for patients with stage I disease. For stage III disease (node-positive), the 5-year survival rate is 20% to 50% with surgical resection alone thus adjuvant therapy is recommended. For patients with stage II disease, the 5-year survival fluctuates between 50% and 65%. Factors other than the stage adversely affect the outcome. These include old age, male gender, performances status, pMMR/MSI-S status, LVI, PNI, pre-operative CEA level greater than 5, poor histological grade, mucinous or signet ring features, the extent of local invasion T4, bowel perforation/obstruction, and insufficient node sampling less than 12. Mutations in KRAS and BRAF have not shown to be a negative prognostic impact in the adjuvant setting. Adjuvant therapy for stage II with high-risk features should be individualized.[20][2][3][21][22]
Adjuvant Therapy
There is no role for adjuvant chemotherapy for patients with Cca stage I. Low-risk stage II Cca patients can opt for surveillance without adjuvant therapy, individualized discussion with medical oncology or enrolled in a clinical trial. Current ASCO, NCCN and ESMO guidelines strongly recommend adjuvant therapy for selected stage II with high-risk/pMMR/MSI-S disease and all stage III (node-positive). For adjuvant therapy Cca, 3-year DFS is an acceptable surrogate marker for 5-year OS recognized by the FDA based on the results of the Adjuvant Colon Cancer End Points study group (ACCENT trial). Guides advocate delivering adjuvant chemotherapy within 6 to 8 weeks of surgical resection pending on patient health recovery. A meta-analysis demonstrated that a 4-week beyond the time to adjuvant chemotherapy was associated with a significant decrease in both overall survivals (HR, 1.14; 95% CI, 1.10-1.17) and disease-free survival (HR, 1.14; 95% CI, 1.10-1.18). An oxaliplatin-based combination is preferred over monotherapy either by FU/LV (MOSAIC trial, NSABP C-07 trial) or capecitabine (XELOXA trial). The standard recommended length of adjuvant therapy is six months and a potentially non-inferiority three months is under current investigation by the International Duration Evaluation of Adjuvant Chemotherapy (IDEA) trial for low-risk patients or oxaliplatin dose-limiting neurotoxicity. The recommendation is to use full weight-based chemotherapy dosing particularly on those with the intention to cure.[23][24][25]
Adjuvant fluorouracil-based chemotherapy became a standard of care in stage III Cca in the early 1990s by the first randomized clinical trial, the National Surgical Adjuvant Breast and Bowel Project (NSABP) C-01, to achieve a significant improvement in DFS and OS. Evidence from a subsequent NSABP C-03 trial demonstrated that fluorouracil (5-FU) combined with leucovorin (LV) provided a superior DFS and OS at three years with six months of adjuvant therapy over NSABP C-01 lomustine, vincristine, and 5-FU. For more than a decade, 5-FU/LV remained the standard in adjuvant therapy until oxaliplatin, irinotecan, and capecitabine (oral FU pro-drug) based combinations were utilized for the treatment of advanced CRC.
Oxaliplatin was approved as part of adjuvant treatment for stage III Cca after the 2004 MOSAIC trial. Investigators randomly assigned patients who had undergone curative colon resection for stage II (40%) or III (60%) to receive FU/LV alone (LV 200 mg/m as a 2-hour infusion, followed by bolus FU 400 mg/m, and then a 22-hour infusion of FU 600 mg/m, every 2 weeks) or with oxaliplatin (FU/LV plus 85 mg/m day 1 every 14 days, a regimen termed FOLFOX4) for 6 months. Adding oxaliplatin (FOLFOX4) significantly improved 5-year DFS rates to 73.3% versus 67.4% FU/LV group, [(HR)= 0.80; 95% CI, 0.68 to 0.93; P = .003] and 6-year OS rates were 78.5% and 76.0% in the FOLFOX4 and FU/LV groups, respectively (HR = 0.84; 95% CI, 0.71 to 1.00; P = .046). Stratified, 6-year OS rates for patients with stage III disease were 72.9% and 68.7%, respectively (HR = 0.80; 95% CI, 0.65 to 0.97; P = .023) but no significant difference in OS was seen in the stage II population. A 10-year OS long-term follow up results corroborated before mentioned trend results. Neuropathy was seen 92% of FOLFOX4 patients, being 13% severe grade and almost all reversible. The United States oncologists had made further modifications to the regimen including FOLFOX6 (single day regimen) and FOLFOX7 (no FU bolus). In a retrospective review of unselected patients with stage II disease, the 5-year DFS benefit for FOLFOX compared with FU/LV alone was no statistically significant (84% versus 80%, HR for recurrence 0.84, p = 0.26), but it significantly exceeded 5% for patients (82% versus 77%) on stage II tumors with high-risk clinical features (poor histological grade, T4, perforation/obstruction, less than 10 lymph nodes sampling, PNI, and LVI).
In 2011, two other trials confirmed the value of oxaliplatin-based regimen as a component of adjuvant chemotherapy for stages II and III colon cancer above FU/LV monotherapy. The NSABP C-07 phase III clinical trial evaluated the impact on DFS of adding oxaliplatin to bolus weekly FU combined with LV as surgical adjuvant therapy for stage II (29%) and III (71%) Cca patients. After intent of curative resection, patients were randomly assigned to either FU (500 mg/m2 IV) bolus weekly for 6 weeks plus LV (500 mg/m2 IV) weekly for 6 weeks during each 8-week cycle for three cycles (FU/LV), or the same FU/LV regimen with oxaliplatin (85 mg/m2 IV) administered on weeks 1, 3, and 5 of each 8-week cycle for three cycles (FLOX). In the NSABP C-07 trial, the 8-year OS was similar between treatment groups (HR, 0.88; 95% CI, 0.75 to 1.02; .08). FLOX remained superior for DFS (HR, 0.82; 95% CI, 0.72 to 0.93; .002). The effect of oxaliplatin on OS did not differ by stage of disease (II versus III = 0.38 for OS; 0.37 for DFS) but did vary by age (less than 70 versus greater than 70 0.39) with a similar trend for DFS (p = 0.073). Oxaliplatin significantly improved OS in patients younger than age 70 (HR, 0.80; 95% CI, 0.68 to 0.95; P = .013), but no positive effect was seen in older patients. FLOX regimen was associated with higher hospitalization for diarrhea (5.5% versus 3%), and a total of 1.2% of patients died of any cause within 60 days of receiving chemotherapy, with no significant difference between regimens.
The XELOXA trial, a multicenter, randomized trial compared capecitabine plus oxaliplatin (XELOX) with bolus FU/LV as adjuvant therapy for patients with stage III Cca. Patients were randomly assigned to XELOX (O: 130 mg/m(2) on day 1 plus X: 1000 mg/m(2) twice daily on days 1 to 14 every 3 weeks for 24 weeks) or the standard bolus FU/LV adjuvant regimen. XELOX improved DFS and OS compared with bolus FU/LV in patients with resected stage III Cca after a median follow-up of almost 7 years (DFS 63% versus 56%, HR, 0.80; 95% CI; 0.69; 0.93; p = 0.0045 and OS 73% versus 67%, HR, 0.83; 95% CI, 0.70 to 0.99; P = .04). XELOX was associated with more neurotoxicity, hand-foot syndrome, and thrombocytopenia. Patients on capecitabine are recommended not to take proton pump inhibitor due to poor absorption of higher gastric pH. Another trial established that six months of oral capecitabine monotherapy is a safe and equally effective alternative to conventional FU/LV for stage III colon cancer.
There is a role for adjuvant chemotherapy for selected patients with stage II high-risk disease. The largest study of Stage II Cca, the UK QUick And Simple And Reliable (QUASAR) trial randomly assigned non-stratified patients (e.g., high-risk and low-risk) to have FU/LV with or without levamisole (later removed) as adjuvant chemotherapy following surgical resection. The relative risk (RR) of death from any cause with chemotherapy versus observation alone was 0.82 (95% CI 0.70 to 0.95; p=0.008) and for recurrence RR 0.78 (0.67-0.91; p=0.001) after ten years follow-up. Although the absolute improvement in survival with FU/LV was 3.6%, the quality of the trial has been question due to a poor median of 6 lymph node dissection (greater than 60% with less than 12 lymph nodes), a group of patients received radiation therapy (14%) or portal infusion chemotherapy (6%), which does not meet current standards. The ACCENT retrospective analysis has demonstrated a trend toward an improved of 10-year DFS and OS for stage II with chemotherapy compared to observation regardless of the risk feature with high-risk disease (51% versus 35%). The identification of high-risk prognostic factors might help distinguish patients at higher risk of relapse which will more likely benefit from adjuvant therapy. Datasets confirmed that patients with stage II Cca without high-risk features and MSI-H/dMMR have an excellent prognosis and do not need to be treated with adjuvant chemotherapy, but remains uncertain for those with high-risk disease.
Irinotecan-based regimens are effective in palliative therapy for advanced CRC, but for unknown reasons, none of the three major phase III trials (CALGB89803, PETACC-3, ACCORD) using combination regimens of irinotecan plus FU/LV has demonstrated to be significantly superior in 3-year DFS when compared with FU/LV alone. The role of novel targeted agents, bevacizumab (antibody against VEGF) and cetuximab (antibody against EGFR), have been investigated in the adjuvant setting in large phase III trials. Three major trials have failed to demonstrate any benefit of adjuvant bevacizumab with FOLFOX (NSABP C-08), XELOX (AVANT) or Capecitabine (QUASAR2). Cetuximab was also tested as a component of adjuvant therapy in stage III Cca added to FOLFOX backbone, unfortunately, failed to improve outcome even on preselected KRAS wild-type tumor, and was detrimental to KRAS-mutated patients. Cetuximab lack of efficacy was later confirmed in the PETACC-8 trial on patients with RAS and BRAF wild-type stage III Cca.
Based on prior clinical trial results, the standard adjuvant chemotherapy for stage III Cca is an oxaliplatin-containing regimen (FOLFOX, XELOX, FLOX) administered for six months. Capecitabine or FU/LV monotherapy should be reserved for patients who are not considered optimal candidates for oxaliplatin (e.g., pre-existing neuropathy). Stage III Cca patient with MSI-high/deficient-MMR tumors are intrinsically resistant to adjuvant FU monotherapy, requiring the addition of oxaliplatin to overcome the resistance and impact outcomes. Patients with Stage II Cca and any high-risk combination should discuss risk and benefit of adjuvant therapy. Retrospective analysis from trials did not document a significant improvement with the addition of oxaliplatin to 5-FU/LV for patients over age 70, and only younger patients had a statistically significant benefit regarding DFS, OS, and time to tumor recurrence. At this point in time, irinotecan-based and targeted therapies have no indication in the adjuvant therapy of Cca outside of investigational trials. There is no conclusive evidence to recommend standard RT alone or combined CRT in the adjuvant setting. There are two web-based validated tools to calculate the relative risk of disease recurrence and mortality based on clinicopathological features and potential adjuvant benefit (an ACCENT by the Mayo Clinic and Adjuvant! Online). Furthermore, gene profiling by Oncotype DX Colon, ColDX or ColoPrint are currently available in clinical practice to assist in risk assessment but has no valid predictive value t, thus not recommended by clinical practice guidelines.
Neoadjuvant Therapy
The role of pre-operative chemotherapy for patients with Cca is currently on a phase III FOxTROT trial (NCT00647530) after promising results of their pilot study. The Foxtrot collaborative group randomized locally advanced resectable (T3 greater than 5 mm invasion beyond muscularis propia and T4) candidates to 3 preoperative cycles of FOLFOX (O; 85 mg/m(2), LV; 175 mg, FU; 400 mg/m(2) bolus, then 2400 mg/m(2) by 46 hour infusion), surgery and nine additional cycles or surgery and 12 adjuvant cycles. Findings favored neo-adjuvant therapy with a tumor regression (31% versus 2%), node-positive (1% versus 20%) and negative margins (4% versus 20%) resulting in a significant downstaging (p=0.04).
Systemic Therapy
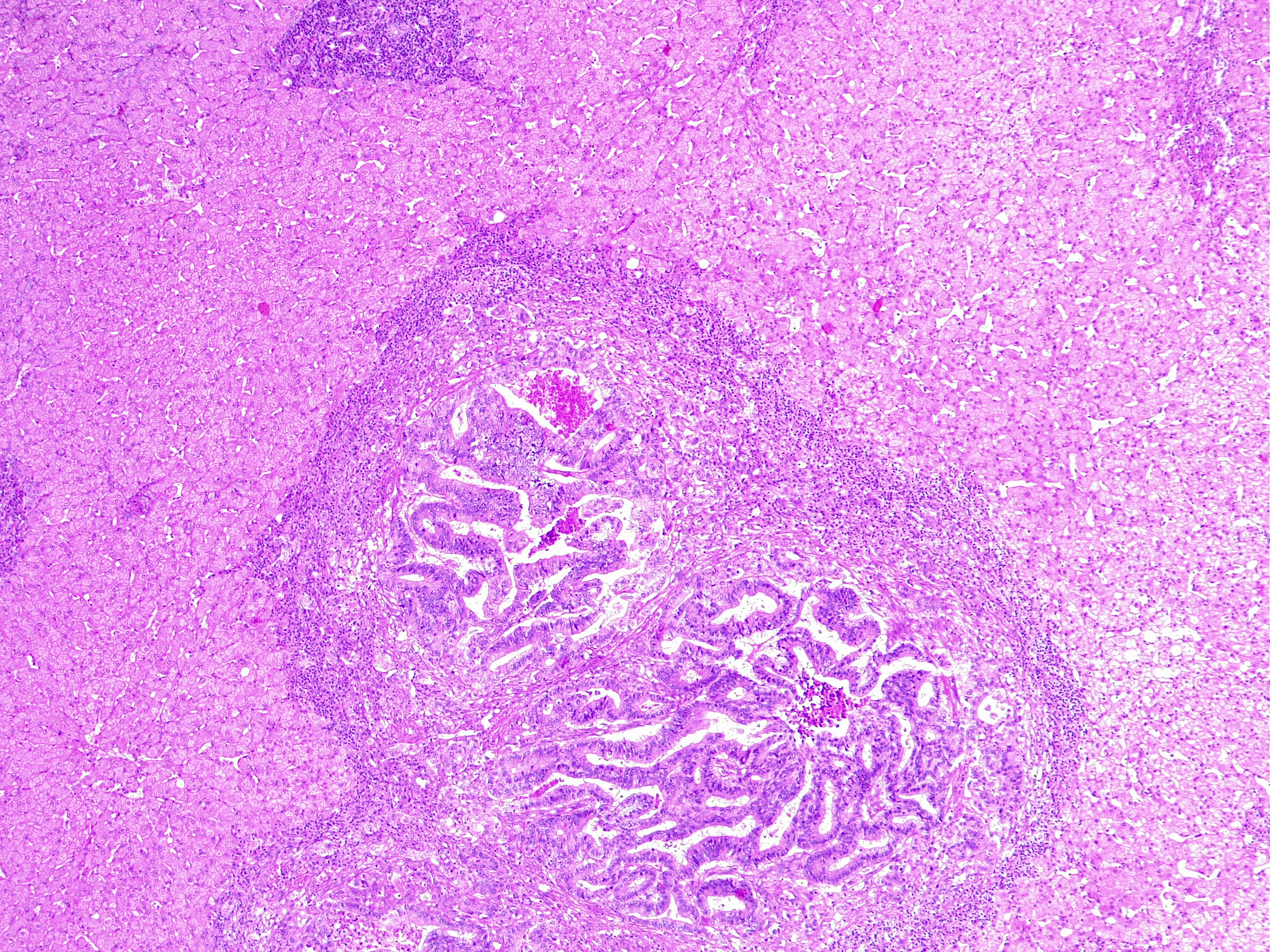
More than half of all CRC patients will develop metastasis and the majority to the liver (80% to 90%). The prognosis for advanced non-resectable and metastatic CRC patients with best supportive care is poor by a mOS 5 to 6 months, except for a subset of patients with oligo-metastatic hepatic or pulmonary patient that are potentially curable with peri-operative chemotherapy. The goals of systemic therapy for advanced non-resectable and metastatic CRC of therapy are palliation of symptoms, improvement of the quality of life and prolong survival. Systemic therapy for cancers of the colon and rectum are addressed jointly by clinical research and practice. In the 1990s, FU/LV monotherapy was the standard first-line therapy with an approximately mOS 12 months. It was until the early 2000s that the addition of oxaliplatin and irinotecan to the backbone of FU/LV resulted in an improvement of mOS to nearly 24 months. The introduction of biologic agents monoclonal antibodies (MAbs) targeting vascular endothelial growth factors (VGFRs) and epidermal growth factors receptor (EGFR) further enhanced the efficacy of systemic medical therapy with mOS reaching 36 months and when exposed to multiple lines of therapy an impressive 20% 5-year OS even at a metastatic stage.
Current availability of nine different antineoplastic classes and more than a dozen drug options fluoropyrimidines (FU), capecitabine (CAP), S-1, tegafur plus uracil (UFT), irinotecan, oxaliplatin, anti-EGFRs (cetuximab, panitumumab), anti-VEGFRs (bevacizumab, ramucirumab), recombinant fusion protein (aflibercept), tyrosine kinase inhibitors (regorafenib), antimetabolites (TAS-102), immunotherapy (nivolumab, pembrolizumab) for mCRC has resulted in an abundance of therapeutic possibilities with different combinations and sequences not yet established. The most appropriate treatment regimen is conceivably the one that generates the highest overall response rate (ORR), greatest impact to metastases for surgical conversion and/or ultimately offers the longest PFS and OS, balanced with a favorable toxicity profile. Predictive biomarker (RAS/BRAF type and MMR/MSI status), primary location (tumor and metastasis), patient (performance status and comorbidities) and therapy goals (palliate or conversion) can help guide treatment decisions for specific patient subpopulations.
For otherwise healthy candidates, the standard first-line treatment approach is to start with a FOLFOX, CAPOX or FOLFIRI regimen plus anti-EGFR (cetuximab or panitumumab) when the tumor is RAS/BRAF wild-type and left-sided or anti-VGFR (bevacizumab) when the tumor is RAS/BRAF mutated or right-sided. At progression of disease or unacceptable toxicity switch to the opposite second-line regimen with either continuation of prior anti-VGFR or new biological agent regardless of the tumor location. ASCO, ESMO, and NCCN recommend comprehensive testing of KRAS, NRAS exons 2 (codons 12 and 13), 3 (codons 59 and 61), 4 (codons 117 and 146) and BRAF V600E for anti-EGFR candidates based the results of the Panitumumab Randomized Trial in Combination with Chemotherapy for Metastatic Colorectal Cancer to Determine Efficacy (PRIME) trial and other major meta-analyses. Biological agents additions to chemotherapy backbone have significantly improved ORR, DFS, and OS in the first and second-line setting. Cetuximab and panitumumab have comparable efficacy in first, second line addition and even single agent salvage therapy, but may share cross-resistance limiting use after progression of anti-EGFRs. Bevacizumab can be continued beyond progression of first-line to second-line therapy (e.g., RAS/BRAF-mutated) but not in conjunction with the new anti-EGFRs candidate (e.g., RAS/BRAF wild-type and left sided). For patients who progressed anti-VEGF bevacizumab may consider aflibercept or ramucirumab addition to backbone chemotherapy regimen.
Sequential single agents remains a valid option for mCRC patients compared to combination treatments demonstrated in the United Kingdom Medical Research Council's FOCUS (Fluorouracil, Oxaliplatin, CPT-11: Use and Sequencing) and the Dutch Colorectal Group CAIRO (capecitabine, irinotecan, oxaliplatin) trials, although biological agents were not used on both trials, and most of all patients did not receive all three drugs. Multiple phase III trials have confirmed similar ORR, PFS, and OS between FOLFOX and FOLFIRI regimens, even with added anti-VEGFs or anti-EGFRs. In clinical practice, the choice between FOLFOX and FOLFIRI is based on treatment toxicity profile. CAPOX combination is a statistically non-inferior substitute to FOLFOX regimen in PFS and OS with tolerable toxicity profile; conversely, XELIRI had shown higher intolerable rates of gastrointestinal toxicities. Maintenance therapy with low dose capecitabine-bevacizumab after the first-line FOLFOX-bevacizumab good response is an accepted treatment option based on CAIRO3 trial results. Complete discontinuation of chemotherapy may be detrimental and remains controversial, but intermittent therapy has not resulted in significant reduced OS but the rather better quality of life.
FOLFOXIRI with or without bevacizumab is reserved for conversion approach in potential resectable liver/lung metastasis on selected excellent performance status patients. Patients not fit for triple or doublet therapies, consensus-based guidelines recommend FU/LV IV or capecitabine PO monotherapy. For patients after FOLFOX, CAPOX, FOLFIRI, anti-VEGFs and anti-EGFRs agent progression that remains eligible for further chemotherapy may consider regorafenib or TAS-102 salvage therapy, or alternative, immunotherapy is approved for MSI-H/dMMR tumors. Novel targeted agents may be available through clinical trials at different lines of therapies failure.
Recommended treatment monitoring consists of close observation of signs/symptoms of adverse reaction (before each treatment cycle and as needed), frequent checking on blood work parameters (before each treatment cycle and as indicated), CEA levels serial assays (every 1 to 3 months) and radiographic evaluation by Response Evaluation Criteria In Solid Tumors (RECIST) (every 2 to 3 months) by a multidisciplinary team. Patients should frequently be screen for somatic symptoms and psychosocial distress.
Selected Landmark Clinical Trials
Cytotoxic Chemotherapy
Fluorouracil-Leucovorin (FU/LV): An updated-meta analysis compared to FU-alone resulted in higher ORR (21% versus 11%) and 1-year OS (47% versus 37%) favoring FU/LV. The two most commonly used regimens in the United States include the Mayo regimen (bolus FU: 425 mg/m2 and LV: 20 mg/m2 on days 1 to 5 every 4 to 5 weeks) and the Roswell Park regimen (Bolus FU: 500 mg/m2 and LV: 500 mg/m2 administered weekly for 6 out of 8 weeks). Further improved by the de Gramont regimen (LV: 200 mg/m2 as a 2-hour infusion followed by bolus FU: 400 mg/m2 and 22-hour infusion FU: 600 mg/m2 for 2 consecutive days every 2 weeks) with significantly better ORR (33% vs 14%), PFS (7 versus 5 months) and OS (15.5 versus 14.2 months) with significantly less grade 3/4 toxicities (23.9% vs 11.1%) compared to the Mayo regimen.
Capecitabine (CAP): A phase III clinical trial by Hoff PM et al. prospectively randomly assigned patients to oral CAP (1250 mg/m2 twice daily for 14 days every 21 days) or FU/LV (the Mayo regimen) resulting in non-inferior ORR (24.8% versus 15.5%), time to progression (TTP 4.3 versus 4.7 months) and mOS (12.5 versus 13.3 months), respectively. Common side effects of CAP included diarrhea (overlapping toxicities with irinotecan), hyperbilirubinemia and hand-foot syndrome. CAP has never been directly compared with infusional FU/LV (de Gramont regimen), CAPOX versus infusional FOLFOX has shown to be of similar efficacy in the treatment of advanced CRC, as shown by the phase TREE-1 trial and AIO trial. In the United States clinical practice, dose-reducing CAP by about 20% (1000 mg/m2) alone or in combination regimens does not appear to decrease the treatment efficacy, but it greatly improves the side effect profile of the treatment. Other alternative oral fluoropyrimidines not approved in the United States include S1-derived three different agents tegafur-uracil, gimeracil and oteracil, and raltitrexed.
Irinotecan (IRI): In a landmark phase II clinical trial, patients with FU-refractory mCRC were randomly assigned either single-agent IRI (300 mg/m2 every 3 weeks) or BSC, showed a significant 1-year OS (36% versus 14%) with improved quality of life (performance status, weight loss, and pain-free), respectively. Following this, three key trials were conducted to test the role of IRI versus FU/LV in the front-line setting. A three-arm trial compared three treatment regimens: the Roswell Park regimen, IRI plus FU/LV weekly bolus regimen (IFL or Saltz regimen); and IRI alone.
The results of the trial significantly favored IFL with an ORR (39% versus 21%) and mOS (14.8 versus 12.6 months). In Europe, three pivotal phase III trials compared the FU/LV regimens to FOLFIRI regimens (the Douillard regimen-IRI: 180 mg/m2 on day 1, FU: 400 mg/m2 bolus followed by 600 mg/m2 over 22 hours, both on days 1 and 2, and LV: 200 mg/m2 on days 1 and 2). The Douillard FOLFIRI regimen trial demonstrated a significant ORR (49% versus 31%), TTP (6.7 versus 4.4 months) and extended mOS (17.4 versus 14.1 months). Higher side effects of irinotecan-group were diarrhea, myelosuppression, and alopecia. Of note, IRI combinations require an adequate biliary function for its active glucuronide metabolite, SN-38, be excreted. Approximately 10% of United States patients are homozygous for the UGT1A1*28 allele polymorphism increasing SN-38 bioavailability and therefore recommended starting with a lower dose of irinotecan.
Oxaliplatin (OXA): OXA has very limited activity in CRC as a single agent thus not recommended unless synergist with fluoropyrimidines. In phase III trial, FU/LV (de Gramont regimen) was compared with or without OXA (OXA: 85 mg/m2 on day 1 over 2 hours FOLFOX4 regimen) as first-line therapy for patients with mCRC. FOLFOX4 regimen had significantly higher ORR (51% versus 22%), PFS (9 versus 6 months) but comparable mOS (16.2 versus 14.7 months) with more grade 3/4 neutropenia, diarrhea, and neurotoxicity. Because no overall survival benefit was achieved in these first-line trials, the FDA did not approve OXA for CRC until years later for second-line therapy that showed prolonged PFS and increased ORR compared with FU/LV for patients who experienced disease progression while receiving first-line IRI-regimens. The most important side effect and dose-limiting toxicity of OXA is neurotoxicity. It may present as an acute and reversible, cold-triggered sensory neuropathy, or a chronic dose-limiting cumulative sensory neurotoxicity.
Irinotecan versus Oxaliplatin-based regimens: After encouraging results of previous trials conducted in the United States and Europe using OXA and IRI, the North Central Cancer Treatment Group (NCCTG)/Intergroup trial N9741 performed a pivotal and practice-changing trial comparing FOLFOX4, the standard combination IFL and new IROX combination (OXA:85 mg/m plus IRI 200 mg/m, both on day 1 every 3 weeks). The N9741 results demonstrated the superiority of FOLFOX compared with IFL and IROX as first-line therapy for mCRC with ORR (45% versus 31% versus 36%), PFS (8.7 versus 6.9 versus 6.7 months) and mOS (19.5 versus 15 versus 17.3 months), respectively. The toxicity profile likewise favored FOLFOX, except for neurotoxicity. FOLFOX emerged as new standard first-line therapy with rapid and widespread adaptation in the United States.
VEGF Inhibitors
Bevacizumab (BEV): In randomized placebo-controlled phase III trial, IFL plus BEV (5 mg/kg every 2 weeks) was assessed in first-line therapy for mCRC. For the first time, the addition of an anti-VEGF inhibitor was validated as an efficacious antineoplastic treatment by significantly improving ORR (45% versus 35%), PFS (10.6 versus 6.2 months), and mOS (20.3 versus 15.6 months). This trial was the first phase III validation of an antiangiogenic agent as an effective treatment option in human malignancy. Subsequently, FOLFOX-BEV also showed to improve mOS in first-line (TREE-2 trial 23.7 versus 18.2 months) and second-line (ECOG 3200 trial 12.9 versus 10.8 months) settings. The AVEX phase III trial selected patients age 70 or older, who were not deemed to be candidates for OXA or IRI-based chemotherapy first-line regimens, to receive CAP regimen alone or with BEV (7.5 mg/kg intravenously on day 1 given every 3 weeks). The study showed a significantly longer mPFS (9.1 versus 5.1 months) and a remarkable improvement in mOS (20.7 versus 16.8 months) for CAP-BEV than with CAP alone, respectively. Prolonged VEGF inhibition with BEV beyond the first-line progression was evaluated by the European TML (ML18147) phase III trial, which randomly assigned to progression within three months to either continue second-line with or without BEV. The mOS, primary endpoint of the study, favored BEV-chemotherapy based continuation 11.2 months versus 9.8 months chemotherapy alone. This effect was confirmed in PFS (5.7 versus 4.1 months) but not in ORR (4% versus 5%). The toxicity profile observed with BEV consists of hypertension, bleeding, gastrointestinal perforations, impaired wound healing and arterial-venous thrombotic events.
Aflibercept (AFL): AFL was evaluated in the second-line setting among patients who had all progressed OXA-based with or without BEV first-line chemotherapy by the placebo-controlled phase III VELOUR trial. Patients randomly selected to receive FOLFIRI with AFL (4 mg/kg IV every 2 weeks) had a significant improvement in ORR (19.8% versus 11.1%), PFS (6.9 versus 4.7 months and mOS (13.5 versus 12.1 months).
The aflibercept arm toxicity profile had an intensified rate of grade 3/4 diarrhea, mucositis, neutropenia, infection, and fatigue plus hypertension, proteinuria, hemorrhage, and arterial-venous thromboembolic events, usually seen in FOLFIRI-BEV second-line.
Ramucirumab (RAM): In a double-blind placebo-controlled phase III RAISE trial, the addition FOLFIRI-RAM (8mg/IV every two weeks) as second-line therapy for patients progressing with a FOLFOX-BEV improved PFS (5.7 versus 4.5 months) and mOS (13.3 versus 11.7 months). Grade 3/4 neutropenia, hypertension, diarrhea and fatigue was worse on the combination arm.
Anti-EGFR Monoclonal Antibodies
Cetuximab (CET): CET monotherapy (400 mg/m2 followed by a weekly infusion of 250 mg/m2) for patients who had experienced disease progression on prior FU, OXA and IRI-based therapy had an improved ORR (40% vs. 11%) and mOS (6.1 versus 4.6 months) compared to BSC. The EPIC and BOND trials proved PFS and ORR superiority of IRI-CET over IRI-alone on patients with prior IRI failure, without significantly improving survival. A large multicenter randomized phase III CRYSTAL trial, FOLFIRI with or without cetuximab in treatment naïve CRC patients, confirmed that selected KRAS wild-type patients have a significantly higher ORR, PFS, and mOS (23.5 versus 20 months). The benefit of FOLFOX-CET remains uncertain with three trials (OPUS, CALBG 80203, CALBG 80405) showing modest significant ORR and PFS improvements but no mOS benefit, and three other trials (MRC COIN, NORDIC VII, New EPOC) with no resulted benefit. CET arm had higher-grade diarrhea, magnesium-wasting syndrome, infusion reaction and ocular-skin toxicity, last correlated to response rate.
Panitumumab (PAN): Single-agent PAN (6 mg/kg every 2 weeks) was compared to BSC in a large international phase III trial in chemotherapy-refractory mCRC patients. PAN had an ORR (37% similar to CET) and modest prolonged mPFS (8 versus 7.3 weeks), but mOS was not increased likely because crossed over from BSC to the PAN arm. The ASPECCT phase III trial compared head-to-head PAN versus CET monotherapies on chemotherapy-refractory patients and showed non-inferior mOS (10 months) with similar expected toxicity profile. FOLFOX4-PAN benefit in first-line setting was seen in phase III PRIME trial with mPFS improvement (9.6 versus 8 months) and trend for mOS (23.0 versus 19.7 months) not significant at 55 weeks follows up.
VEGF Inhibitors Versus Anti-EGFR Monoclonal Antibodies
The FIRE-3 trial recruited treatment naïve mCRC KRAS exon2 wild-type patients and randomly assigned to receive FOLFIRI_CET or FOLFIRI-BEV. The primary endpoint of the trial, objective response rate analyzed by intention-to-treat analysis, was not reached (CET 62% versus BEV 58%, p = 0.18). Although no difference in PFS was noted (CET 10 versus BEV 10.3 months), the mOS was significantly longer in the FOLFIRI-CET arm (CET 28.7 versus BEV 25.0 months; HR, 0.77; p = 0.017). An updated analysis, which accounted for additional mutations in KRAS exon 3 and 4, as well as NRAS mutations exon 2 and 3, demonstrated a longer mOS survival of 33.1 months for FOLFIRI-CET. In contrast, the U.S. Intergroup study, CALGB/South-west Oncology Group (SWOG) 80405 trial that compared FOLFOX or FOLFIRI (dealer’s choice) with CET compared with BEV as first-line therapy had no difference in mOS (30 versus 29.9 months, respectively), not even whit expanded RAS-mutated analysis. The preliminary retrospective analysis suggests that patients treated with CET for KRAS wild-type left-sided tumors mOS are significantly higher than right-sided counterpart by 33.3 versus 19.4 months, respectively. Combination therapy with VEGF inhibitors and anti-EGFRs monoclonal antibody have failed even detrimental in multiple trials (BOND-2, PACCE, CAIRO-2), thus not recommended.
Salvage Therapy for Refractory Disease
Regorafenib (REG): REG (160mg PO daily for three weeks of 4 weeks cycle) efficacy was investigated in a placebo-controlled, multicenter international, randomized, phase III CORRECT trial after progression of multiple therapies. REG compared with placebo had ORR (41% versus 15%), PFS (1.9 versus 1.7 months) and modest mOS benefit (6.4 versus 5.0 months) but significant (p = 0.0052). The observed toxicity profile with regorafenib was hand-foot skin reaction, fatigue, hypertension, diarrhea, and rash, with a 1.6% fatal hepatic failure. The CONCUR trial later confirmed REG benefit.
Trifluridine-tipiracil (TAS-102): the international, double-blind, placebo-controlled randomized-controlled, phase III RECOURSE study of patients with refractory showed that TAS-102 (35 mg/m2 orally twice daily on days 1 through 5 and 8 to 12 of each 28-day cycle) improved ORR (44% versus 16%), DFS and mOS (7.1 versus 5.3 months); The most adverse event was neutropenia (38%), febrile neutropenia (4%) and one treatment-related death.
Immune Checkpoint Inhibitors
Pembrolizumab (PEM): In a phase II study, PEM (10 mg/kg every 14 days) was given to 11 heavily pretreated patients with dMMR mCRC with a remarkable ORR of 71% and PFS rate of 67%. An expanded cohort of 54 patients, PEM showed OOR of 50% and an 89% disease control rate with the durable response for more than a year, not seen in pMMR CRC.
Nivolumab (NIV): In CheckMate-142, NIV (3mg/kg every 2 weeks) was given to 74 dMMR CRC patients resulted in 31% objective response, 69% disease control, and median duration response not reached at 12 months.