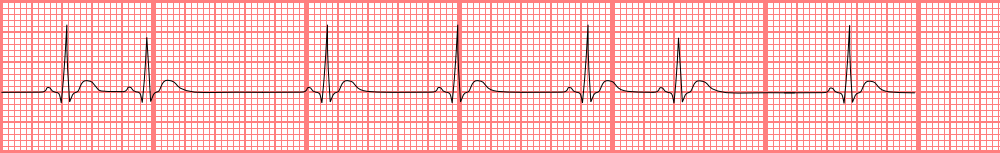
[1]
Lazzeroni E, Domenicucci S, Finardi A, Zoni A, Dodi C, Francescon P, Botti G. Severity of arrhythmias and extent of hypertrophy in hypertrophic cardiomyopathy. American heart journal. 1989 Oct:118(4):734-8
[PubMed PMID: 2529748]
[2]
Ramsdale DR, Arumugam N, Singh SS, Pearson J, Charles RG. Holter monitoring in patients with mitral stenosis and sinus rhythm. European heart journal. 1987 Feb:8(2):164-70
[PubMed PMID: 2436916]
[3]
Ergül Y, Öztürk E, Özgür S. Successful radiofrequency ablation of accessory pathway associated with left atrial appendage aneurysm in a low birthweight premature patient. The Turkish journal of pediatrics. 2019:61(1):142-146. doi: 10.24953/turkjped.2019.01.025. Epub
[PubMed PMID: 31559738]
[4]
Durmaz E,Ikitimur B,Kilickiran Avci B,Atıcı A,Yurtseven E,Tokdil H,Ebren C,Polat F,Karaca O,Karadag B,Ongen Z, The clinical significance of premature atrial contractions: How frequent should they become predictive of new-onset atrial fibrillation. Annals of noninvasive electrocardiology : the official journal of the International Society for Holter and Noninvasive Electrocardiology, Inc. 2019 Oct 11;
[PubMed PMID: 31603280]
[5]
Pascall E, Tulloh RM. Pulmonary hypertension in congenital heart disease. Future cardiology. 2018 Jul:14(4):343-353. doi: 10.2217/fca-2017-0065. Epub 2018 May 24
[PubMed PMID: 29792339]
[6]
Savio-Galimberti E, Argenziano M, Antzelevitch C. Cardiac Arrhythmias Related to Sodium Channel Dysfunction. Handbook of experimental pharmacology. 2018:246():331-354. doi: 10.1007/164_2017_43. Epub
[PubMed PMID: 28965168]
[7]
Czaja AS, Ross ME, Liu W, Fiks AG, Localio R, Wasserman RC, Grundmeier RW, Adams WG, Comparative Effectiveness Research through Collaborative Electronic Reporting (CER2) Consortium. Electronic health record (EHR) based postmarketing surveillance of adverse events associated with pediatric off-label medication use: A case study of short-acting beta-2 agonists and arrhythmias. Pharmacoepidemiology and drug safety. 2018 Jul:27(7):815-822. doi: 10.1002/pds.4562. Epub 2018 May 27
[PubMed PMID: 29806185]
Level 3 (low-level) evidence
[8]
Kamineni P, Prakasa K, Hasan SP, Akula R, Dawkins F. Cardiotoxicities of paclitaxel in African Americans. Journal of the National Medical Association. 2003 Oct:95(10):977-81
[PubMed PMID: 14620711]
[10]
Holtzman D, Aronow WS, Mellana WM, Sharma M, Mehta N, Lim J, Chandy D. Electrocardiographic abnormalities in patients with severe versus mild or moderate chronic obstructive pulmonary disease followed in an academic outpatient pulmonary clinic. Annals of noninvasive electrocardiology : the official journal of the International Society for Holter and Noninvasive Electrocardiology, Inc. 2011 Jan:16(1):30-2. doi: 10.1111/j.1542-474X.2010.00404.x. Epub
[PubMed PMID: 21251131]
[11]
Terasaki F, Kitaura Y, Hayashi T, Nakayama Y, Deguchi H, Kawamura K. Arrhythmias in Coxsackie B3 virus myocarditis. Continuous electrocardiography in conscious mice and histopathology of the heart with special reference to the conduction system. Heart and vessels. Supplement. 1990:5():45-50
[PubMed PMID: 1965542]
[12]
Wang Z, Qin H, Chen G, Dai Y, Cai Y, Cheng X, Qian Y, Chu M, Lu X. Anxiety is associated with increased risk for atrial cardiopathy. Acta neurologica Belgica. 2020 Dec:120(6):1383-1388. doi: 10.1007/s13760-020-01335-0. Epub 2020 Mar 19
[PubMed PMID: 32193730]
Level 2 (mid-level) evidence
[13]
Shotan A, Ostrzega E, Mehra A, Johnson JV, Elkayam U. Incidence of arrhythmias in normal pregnancy and relation to palpitations, dizziness, and syncope. The American journal of cardiology. 1997 Apr 15:79(8):1061-4
[PubMed PMID: 9114764]
[14]
Stamler JS, Goldman ME, Gomes J, Matza D, Horowitz SF. The effect of stress and fatigue on cardiac rhythm in medical interns. Journal of electrocardiology. 1992 Oct:25(4):333-8
[PubMed PMID: 1402519]
[15]
Day E, Rudd JHF. Alcohol use disorders and the heart. Addiction (Abingdon, England). 2019 Sep:114(9):1670-1678. doi: 10.1111/add.14703. Epub 2019 Jul 15
[PubMed PMID: 31309639]
[16]
Kamel H, Pearce LA, Ntaios G, Gladstone DJ, Perera K, Roine RO, Meseguer E, Shoamanesh A, Berkowitz SD, Mundl H, Sharma M, Connolly SJ, Hart RG, Healey JS. Atrial Cardiopathy and Nonstenosing Large Artery Plaque in Patients With Embolic Stroke of Undetermined Source. Stroke. 2020 Mar:51(3):938-943. doi: 10.1161/STROKEAHA.119.028154. Epub 2020 Jan 2
[PubMed PMID: 31893985]
[17]
Dixit S, Stein PK, Dewland TA, Dukes JW, Vittinghoff E, Heckbert SR, Marcus GM. Consumption of Caffeinated Products and Cardiac Ectopy. Journal of the American Heart Association. 2016 Jan 26:5(1):. doi: 10.1161/JAHA.115.002503. Epub 2016 Jan 26
[PubMed PMID: 26813889]
[18]
Conen D, Adam M, Roche F, Barthelemy JC, Felber Dietrich D, Imboden M, Künzli N, von Eckardstein A, Regenass S, Hornemann T, Rochat T, Gaspoz JM, Probst-Hensch N, Carballo D. Premature atrial contractions in the general population: frequency and risk factors. Circulation. 2012 Nov 6:126(19):2302-8. doi: 10.1161/CIRCULATIONAHA.112.112300. Epub 2012 Oct 9
[PubMed PMID: 23048073]
[19]
Southall DP, Richards J, Mitchell P, Brown DJ, Johnston PG, Shinebourne EA. Study of cardiac rhythm in healthy newborn infants. British heart journal. 1980 Jan:43(1):14-20
[PubMed PMID: 7356857]
[20]
Martin GR, Ruckman RN. Fetal echocardiography: a large clinical experience and follow-up. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 1990 Jan-Feb:3(1):4-8
[PubMed PMID: 2310591]
[21]
Folarin VA, Fitzsimmons PJ, Kruyer WB. Holter monitor findings in asymptomatic male military aviators without structural heart disease. Aviation, space, and environmental medicine. 2001 Sep:72(9):836-8
[PubMed PMID: 11565820]
[22]
Sliwa K, Azibani F, Johnson MR, Viljoen C, Baard J, Osman A, Briton O, Ntsekhe M, Chin A. Effectiveness of Implanted Cardiac Rhythm Recorders With Electrocardiographic Monitoring for Detecting Arrhythmias in Pregnant Women With Symptomatic Arrhythmia and/or Structural Heart Disease: A Randomized Clinical Trial. JAMA cardiology. 2020 Apr 1:5(4):458-463. doi: 10.1001/jamacardio.2019.5963. Epub
[PubMed PMID: 32074256]
Level 1 (high-level) evidence
[23]
Todo K, Iwata T, Doijiri R, Yamagami H, Morimoto M, Hashimoto T, Sonoda K, Yamazaki H, Koge J, Okazaki S, Sasaki T, Mochizuki H. Frequent Premature Atrial Contractions in Cryptogenic Stroke Predict Atrial Fibrillation Detection with Insertable Cardiac Monitoring. Cerebrovascular diseases (Basel, Switzerland). 2020:49(2):144-150. doi: 10.1159/000505958. Epub 2020 Feb 5
[PubMed PMID: 32023609]
[24]
Karunadas CP, Mathew C. Comparison of arrhythmia detection by conventional Holter and a novel ambulatory ECG system using patch and Android App, over 24 h period. Indian pacing and electrophysiology journal. 2020 Mar-Apr:20(2):49-53. doi: 10.1016/j.ipej.2019.12.013. Epub 2019 Dec 19
[PubMed PMID: 31866554]
[25]
Larsen BS, Kumarathurai P, Nielsen OW, Sajadieh A. The circadian variation of premature atrial contractions. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2016 Oct:18(10):1573-1580
[PubMed PMID: 26705556]
[26]
Lown M,Brown M,Brown C,Yue AM,Shah BN,Corbett SJ,Lewith G,Stuart B,Moore M,Little P, Machine learning detection of Atrial Fibrillation using wearable technology. PloS one. 2020;
[PubMed PMID: 31978173]
[27]
Al-Alusi MA, Ding E, McManus DD, Lubitz SA. Wearing Your Heart on Your Sleeve: the Future of Cardiac Rhythm Monitoring. Current cardiology reports. 2019 Nov 25:21(12):158. doi: 10.1007/s11886-019-1223-8. Epub 2019 Nov 25
[PubMed PMID: 31768764]
[28]
Engdahl J, Svennberg E. [The core of the Apple Heart Study]. Lakartidningen. 2020 Feb 17:117():. pii: FYY6. Epub 2020 Feb 17
[PubMed PMID: 32068877]
[29]
Cho DJ. Editoral commentary: Beyond the early adopter: The smartwatch ECG goes mainstream. Trends in cardiovascular medicine. 2020 Oct:30(7):449-450. doi: 10.1016/j.tcm.2019.11.003. Epub 2019 Nov 20
[PubMed PMID: 31810859]
[30]
Kim GE, Ross JL, Xie C, Su KN, Zaha VG, Wu X, Palmeri M, Ashraf M, Akar JG, Russell KS, Akar FG, Young LH. LKB1 deletion causes early changes in atrial channel expression and electrophysiology prior to atrial fibrillation. Cardiovascular research. 2015 Oct 1:108(1):197-208. doi: 10.1093/cvr/cvv212. Epub
[PubMed PMID: 26378152]
[31]
Ikeda Y, Sato K, Pimentel DR, Sam F, Shaw RJ, Dyck JR, Walsh K. Cardiac-specific deletion of LKB1 leads to hypertrophy and dysfunction. The Journal of biological chemistry. 2009 Dec 18:284(51):35839-49. doi: 10.1074/jbc.M109.057273. Epub
[PubMed PMID: 19828446]
[32]
Ozcan C, Battaglia E, Young R, Suzuki G. LKB1 knockout mouse develops spontaneous atrial fibrillation and provides mechanistic insights into human disease process. Journal of the American Heart Association. 2015 Mar 15:4(3):e001733. doi: 10.1161/JAHA.114.001733. Epub 2015 Mar 15
[PubMed PMID: 25773299]
[33]
Choy L, Yeo JM, Tse V, Chan SP, Tse G. Cardiac disease and arrhythmogenesis: Mechanistic insights from mouse models. International journal of cardiology. Heart & vasculature. 2016 Sep:12():1-10
[PubMed PMID: 27766308]
[34]
Vervueren PL,Delmas C,Berry M,Rollin A,Sadron M,Duparc A,Mondoly P,Honton B,Lairez O,Maury P, Reversal of Dilated Cardiomyopathy After Successful Radio-Frequency Ablation of Frequent Atrial Premature Beats, a New Cause for Arrhythmia-Induced Cardiomyopathy. Journal of atrial fibrillation. 2012 Dec;
[PubMed PMID: 28496791]
[35]
Bagliani G, Della Rocca DG, De Ponti R, Capucci A, Padeletti M, Natale A. Ectopic Beats: Insights from Timing and Morphology. Cardiac electrophysiology clinics. 2018 Jun:10(2):257-275. doi: 10.1016/j.ccep.2018.02.013. Epub
[PubMed PMID: 29784483]
[36]
Arif M, Laidlaw JC, Oshrain C, Willis PW 3rd, Nissen CH, McDermott DJ, Smith WS, Karim A, Wilson RR. A randomized, double-blind, parallel group comparison of disopyramide phosphate and quinidine in patients with cardiac arrhythmias. Angiology. 1983 Jun:34(6):393-400
[PubMed PMID: 6408949]
Level 1 (high-level) evidence
[37]
Kimura E, Mashima S, Tanaka T. Clinical evaluation of antiarrhythmic effects of disopyramide by multiclinical controlled double-blind methods. International journal of clinical pharmacology, therapy, and toxicology. 1980:18(8):338-43
[PubMed PMID: 7409935]
Level 1 (high-level) evidence
[38]
Mahtani AU, Nair DG. Supraventricular Tachycardia. The Medical clinics of North America. 2019 Sep:103(5):863-879. doi: 10.1016/j.mcna.2019.05.007. Epub
[PubMed PMID: 31378331]
[39]
Sindby JE, Vadmann H, Lundbye-Christensen S, Riahi S, Hjortshøj S, Boersma LVA, Andreasen JJ. Percutaneous versus thoracoscopic ablation of symptomatic paroxysmal atrial fibrillation: a randomised controlled trial - the FAST II study. Journal of cardiothoracic surgery. 2018 Oct 3:13(1):101. doi: 10.1186/s13019-018-0792-8. Epub 2018 Oct 3
[PubMed PMID: 30285795]
Level 1 (high-level) evidence
[40]
Wang X, Li Z, Mao J, He B. Electrophysiological features and catheter ablation of symptomatic frequent premature atrial contractions. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2017 Sep 1:19(9):1535-1541. doi: 10.1093/europace/euw152. Epub
[PubMed PMID: 27702869]
[41]
Huang X, Chen Y, Xiao J, Zhao H, Chen Y, Liu S, He L, Huang Z, Zhou H, Xu D, Peng J. Electrophysiological characteristics and catheter ablation of symptomatic focal premature atrial contractions originating from pulmonary veins and non-pulmonary veins. Clinical cardiology. 2018 Jan:41(1):74-80. doi: 10.1002/clc.22853. Epub 2018 Jan 25
[PubMed PMID: 29369366]
[42]
Kliś M, Sławuta A, Gajek J. Antiarrhythmic properties of atrial pacing. Advances in clinical and experimental medicine : official organ Wroclaw Medical University. 2017 Mar-Apr:26(2):351-357. doi: 10.17219/acem/61429. Epub
[PubMed PMID: 28791857]
Level 3 (low-level) evidence
[43]
Qureshi W, Shah AJ, Salahuddin T, Soliman EZ. Long-term mortality risk in individuals with atrial or ventricular premature complexes (results from the Third National Health and Nutrition Examination Survey). The American journal of cardiology. 2014 Jul 1:114(1):59-64. doi: 10.1016/j.amjcard.2014.04.005. Epub 2014 Apr 16
[PubMed PMID: 24819898]
Level 3 (low-level) evidence
[44]
Lin CY, Lin YJ, Chen YY, Chang SL, Lo LW, Chao TF, Chung FP, Hu YF, Chong E, Cheng HM, Tuan TC, Liao JN, Chiou CW, Huang JL, Chen SA. Prognostic Significance of Premature Atrial Complexes Burden in Prediction of Long-Term Outcome. Journal of the American Heart Association. 2015 Aug 27:4(9):e002192. doi: 10.1161/JAHA.115.002192. Epub 2015 Aug 27
[PubMed PMID: 26316525]
[45]
Prasitlumkum N, Rattanawong P, Limpruttidham N, Kanitsoraphan C, Sirinvaravong N, Suppakitjanusant P, Chongsathidkiet P, Chung EH. Frequent premature atrial complexes as a predictor of atrial fibrillation: Systematic review and meta-analysis. Journal of electrocardiology. 2018 Sep-Oct:51(5):760-767. doi: 10.1016/j.jelectrocard.2018.05.012. Epub 2018 May 23
[PubMed PMID: 30177309]
Level 1 (high-level) evidence
[46]
Alhede C, Lauridsen TK, Johannessen A, Dixen U, Jensen JS, Raatikainen P, Hindricks G, Walfridsson H, Kongstad O, Pehrson S, Englund A, Hartikainen J, Hansen PS, Nielsen JC, Jons C. The impact of supraventricular ectopic complexes in different age groups and risk of recurrent atrial fibrillation after antiarrhythmic medication or catheter ablation. International journal of cardiology. 2018 Jan 1:250():122-127. doi: 10.1016/j.ijcard.2017.09.208. Epub 2017 Oct 9
[PubMed PMID: 29050922]
[47]
Acharya T, Tringali S, Bhullar M, Nalbandyan M, Ilineni VK, Carbajal E, Deedwania P. Frequent Atrial Premature Complexes and Their Association With Risk of Atrial Fibrillation. The American journal of cardiology. 2015 Dec 15:116(12):1852-7. doi: 10.1016/j.amjcard.2015.09.025. Epub 2015 Oct 3
[PubMed PMID: 26611122]
[48]
Chong BH, Pong V, Lam KF, Liu S, Zuo ML, Lau YF, Lau CP, Tse HF, Siu CW. Frequent premature atrial complexes predict new occurrence of atrial fibrillation and adverse cardiovascular events. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2012 Jul:14(7):942-7. doi: 10.1093/europace/eur389. Epub 2011 Dec 19
[PubMed PMID: 22183750]
[49]
Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DS, Stein PK, Psaty BM, Sotoodehnia N, Gottdiener JS, Marcus GM. Atrial ectopy as a predictor of incident atrial fibrillation: a cohort study. Annals of internal medicine. 2013 Dec 3:159(11):721-8. doi: 10.7326/0003-4819-159-11-201312030-00004. Epub
[PubMed PMID: 24297188]
[50]
Huang BT, Huang FY, Peng Y, Liao YB, Chen F, Xia TL, Pu XB, Chen M. Relation of premature atrial complexes with stroke and death: Systematic review and meta-analysis. Clinical cardiology. 2017 Nov:40(11):962-969. doi: 10.1002/clc.22780. Epub 2017 Aug 28
[PubMed PMID: 28846809]
Level 1 (high-level) evidence
[51]
Gladstone DJ, Dorian P, Spring M, Panzov V, Mamdani M, Healey JS, Thorpe KE, EMBRACE Steering Committee and Investigators. Atrial premature beats predict atrial fibrillation in cryptogenic stroke: results from the EMBRACE trial. Stroke. 2015 Apr:46(4):936-41. doi: 10.1161/STROKEAHA.115.008714. Epub 2015 Feb 19
[PubMed PMID: 25700289]
[52]
Cheriyath P, He F, Peters I, Li X, Alagona P Jr, Wu C, Pu M, Cascio WE, Liao D. Relation of atrial and/or ventricular premature complexes on a two-minute rhythm strip to the risk of sudden cardiac death (the Atherosclerosis Risk in Communities [ARIC] study). The American journal of cardiology. 2011 Jan 15:107(2):151-5. doi: 10.1016/j.amjcard.2010.09.002. Epub
[PubMed PMID: 21211594]