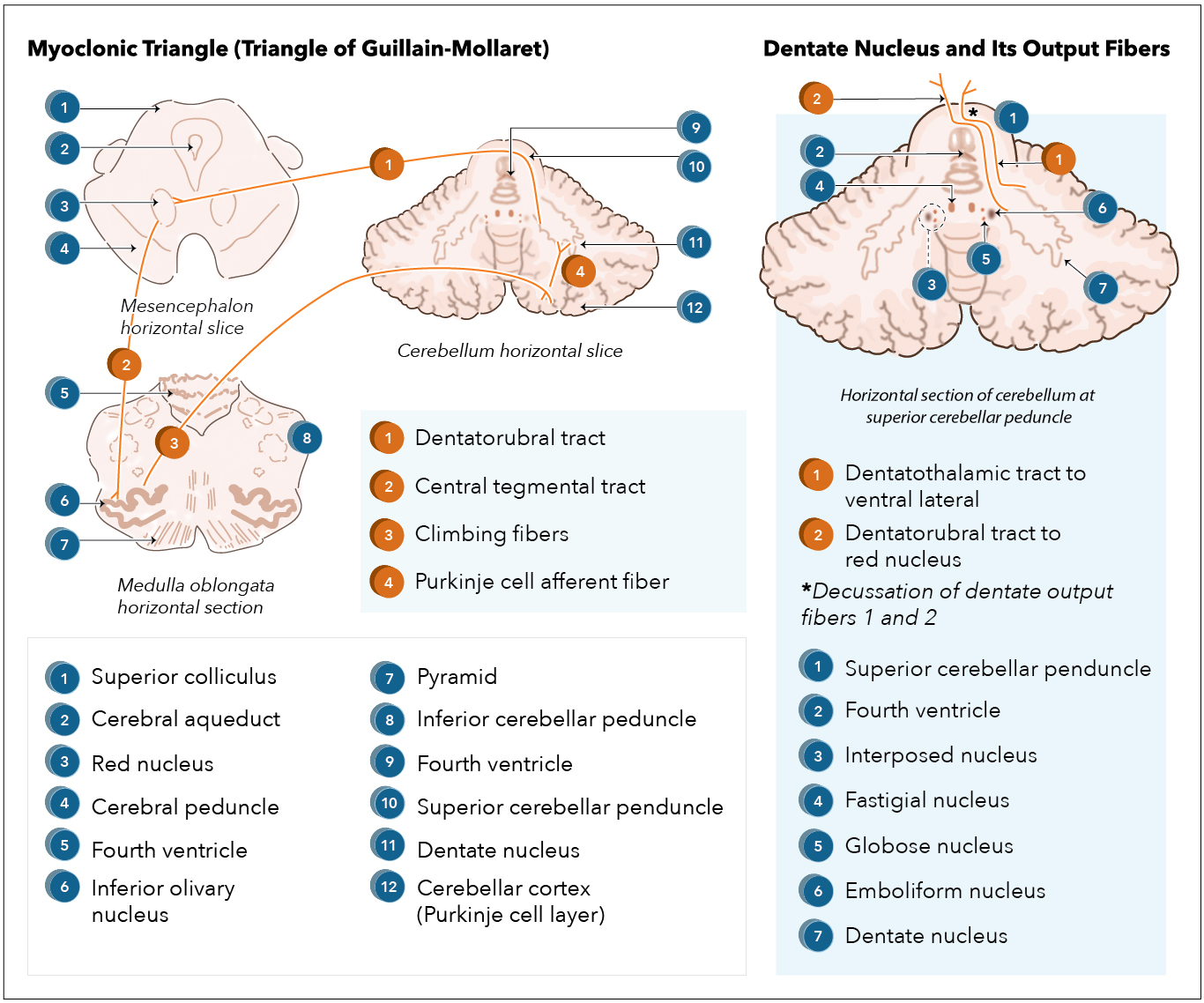
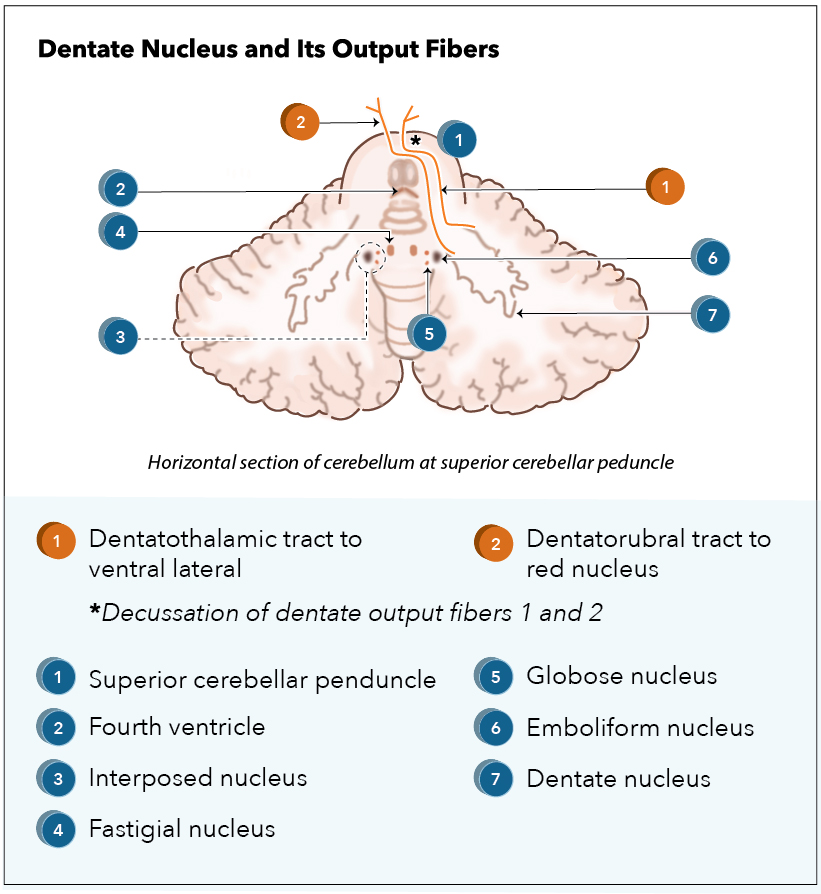
[1]
Bond KM, Brinjikji W, Eckel LJ, Kallmes DF, McDonald RJ, Carr CM. Dentate Update: Imaging Features of Entities That Affect the Dentate Nucleus. AJNR. American journal of neuroradiology. 2017 Aug:38(8):1467-1474. doi: 10.3174/ajnr.A5138. Epub 2017 Apr 13
[PubMed PMID: 28408628]
[2]
Matano S. Brief communication: Proportions of the ventral half of the cerebellar dentate nucleus in humans and great apes. American journal of physical anthropology. 2001 Feb:114(2):163-5
[PubMed PMID: 11169906]
[3]
Barkovich AJ, Millen KJ, Dobyns WB. A developmental and genetic classification for midbrain-hindbrain malformations. Brain : a journal of neurology. 2009 Dec:132(Pt 12):3199-230. doi: 10.1093/brain/awp247. Epub
[PubMed PMID: 19933510]
[4]
Butts T, Green MJ, Wingate RJ. Development of the cerebellum: simple steps to make a 'little brain'. Development (Cambridge, England). 2014 Nov:141(21):4031-41. doi: 10.1242/dev.106559. Epub
[PubMed PMID: 25336734]
[5]
Rahimi-Balaei M, Bergen H, Kong J, Marzban H. Neuronal Migration During Development of the Cerebellum. Frontiers in cellular neuroscience. 2018:12():484. doi: 10.3389/fncel.2018.00484. Epub 2018 Dec 17
[PubMed PMID: 30618631]
[6]
Barthelery NJ, Manfredi JJ. Cerebellum Development and Tumorigenesis: A p53-Centric Perspective. Trends in molecular medicine. 2016 May:22(5):404-413. doi: 10.1016/j.molmed.2016.03.006. Epub 2016 Apr 13
[PubMed PMID: 27085812]
Level 3 (low-level) evidence
[7]
Lee GH, D'Arcangelo G. New Insights into Reelin-Mediated Signaling Pathways. Frontiers in cellular neuroscience. 2016:10():122. doi: 10.3389/fncel.2016.00122. Epub 2016 May 9
[PubMed PMID: 27242434]
[8]
Kim S, Lee H, Lee Y, Lee J, Yang J, Lee M, Yang H. Blood Supply by the Superior Cerebellar Artery and Posterior Inferior Cerebellar Artery to the Motor and Nonmotor Domains of the Human Dentate Nucleus. World neurosurgery. 2019 Feb:122():e606-e611. doi: 10.1016/j.wneu.2018.10.111. Epub 2018 Oct 26
[PubMed PMID: 31108077]
[9]
Tubbs RS, Shoja MM, Aggarwal A, Gupta T, Loukas M, Sahni D, Ansari SF, Cohen-Gadol AA. Choroid plexus of the fourth ventricle: Review and anatomic study highlighting anatomical variations. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2016 Apr:26():79-83. doi: 10.1016/j.jocn.2015.10.006. Epub 2015 Dec 7
[PubMed PMID: 26675624]
[10]
Sakka L, Coll G, Chazal J. Anatomy and physiology of cerebrospinal fluid. European annals of otorhinolaryngology, head and neck diseases. 2011 Dec:128(6):309-16. doi: 10.1016/j.anorl.2011.03.002. Epub 2011 Nov 18
[PubMed PMID: 22100360]
[11]
Dum RP, Strick PL. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. Journal of neurophysiology. 2003 Jan:89(1):634-9
[PubMed PMID: 12522208]
[12]
Bostan AC, Strick PL. The basal ganglia and the cerebellum: nodes in an integrated network. Nature reviews. Neuroscience. 2018 Jun:19(6):338-350. doi: 10.1038/s41583-018-0002-7. Epub
[PubMed PMID: 29643480]
[13]
Koeppen AH. The neuropathology of the adult cerebellum. Handbook of clinical neurology. 2018:154():129-149. doi: 10.1016/B978-0-444-63956-1.00008-4. Epub
[PubMed PMID: 29903436]
[14]
Soteropoulos DS, Baker SN. Bilateral representation in the deep cerebellar nuclei. The Journal of physiology. 2008 Feb 15:586(4):1117-36. doi: 10.1113/jphysiol.2007.144220. Epub 2008 Jan 10
[PubMed PMID: 18187463]
[15]
Allen JS, Damasio H, Grabowski TJ. Normal neuroanatomical variation in the human brain: an MRI-volumetric study. American journal of physical anthropology. 2002 Aug:118(4):341-58
[PubMed PMID: 12124914]
[16]
Vilis T, Hore J. Effects of changes in mechanical state of limb on cerebellar intention tremor. Journal of neurophysiology. 1977 Sep:40(5):1214-24
[PubMed PMID: 409809]
[17]
Arora R. Imaging spectrum of cerebellar pathologies: a pictorial essay. Polish journal of radiology. 2015:80():142-50. doi: 10.12659/PJR.892878. Epub 2015 Mar 16
[PubMed PMID: 25806100]
[18]
Solbach K, Kraff O, Minnerop M, Beck A, Schöls L, Gizewski ER, Ladd ME, Timmann D. Cerebellar pathology in Friedreich's ataxia: atrophied dentate nuclei with normal iron content. NeuroImage. Clinical. 2014:6():93-9. doi: 10.1016/j.nicl.2014.08.018. Epub 2014 Aug 23
[PubMed PMID: 25379420]
[19]
Deuschl G, Toro C, Hallett M. Symptomatic and essential palatal tremor. 2. Differences of palatal movements. Movement disorders : official journal of the Movement Disorder Society. 1994 Nov:9(6):676-8
[PubMed PMID: 7845410]
[20]
Blackburn PR, Gass JM, Vairo FPE, Farnham KM, Atwal HK, Macklin S, Klee EW, Atwal PS. Maple syrup urine disease: mechanisms and management. The application of clinical genetics. 2017:10():57-66. doi: 10.2147/TACG.S125962. Epub 2017 Sep 6
[PubMed PMID: 28919799]
[21]
Steinlin M, Blaser S, Boltshauser E. Cerebellar involvement in metabolic disorders: a pattern-recognition approach. Neuroradiology. 1998 Jun:40(6):347-54
[PubMed PMID: 9689620]
[22]
Hoshino H, Kubota M. Canavan disease: clinical features and recent advances in research. Pediatrics international : official journal of the Japan Pediatric Society. 2014 Aug:56(4):477-83. doi: 10.1111/ped.12422. Epub
[PubMed PMID: 24977939]
Level 3 (low-level) evidence
[23]
Porto L, Schöning S, Hattingen E, Sörensen J, Jurcoane A, Lehrnbecher T. Central nervous system imaging in childhood Langerhans cell histiocytosis - a reference center analysis. Radiology and oncology. 2015 Sep:49(3):242-9. doi: 10.1515/raon-2015-0024. Epub 2015 Aug 21
[PubMed PMID: 26401129]
[24]
Chen J, Cohen ML, Lerner AJ, Yang Y, Herrup K. DNA damage and cell cycle events implicate cerebellar dentate nucleus neurons as targets of Alzheimer's disease. Molecular neurodegeneration. 2010 Dec 20:5():60. doi: 10.1186/1750-1326-5-60. Epub 2010 Dec 20
[PubMed PMID: 21172027]
[25]
Hampson DR, Blatt GJ. Autism spectrum disorders and neuropathology of the cerebellum. Frontiers in neuroscience. 2015:9():420. doi: 10.3389/fnins.2015.00420. Epub 2015 Nov 6
[PubMed PMID: 26594141]
[26]
Tikoo S, Pietracupa S, Tommasin S, Bologna M, Petsas N, Bharti K, Berardelli A, Pantano P. Functional disconnection of the dentate nucleus in essential tremor. Journal of neurology. 2020 May:267(5):1358-1367. doi: 10.1007/s00415-020-09711-9. Epub 2020 Jan 23
[PubMed PMID: 31974808]
[27]
Smith TE, Steven A, Bagert BA. Gadolinium Deposition in Neurology Clinical Practice. The Ochsner journal. 2019 Spring:19(1):17-25. doi: 10.31486/toj.18.0111. Epub
[PubMed PMID: 30983897]