Continuing Education Activity
Hyperkeratosis refers to the increased thickness of the stratum corneum, the outer layer of the skin. It is most frequently due to chronic physical or chemical damage such as friction or the use of aggressive soaps but can also derive from chronic inflammation or a side-effect of different drugs, including chemotherapy. This activity reviews the evaluation and treatment of hyperkeratosis and highlights the role of the interprofessional team in evaluating and treating patients with this condition.
Objectives:
- Identify the etiology of hyperkeratosis.
- Summarize the major histologic forms of hyperkeratosis.
- Review the different pathologies that can manifest with hyperkeratosis.
- Explain the importance of collaboration and communication amongst the interprofessional team to ensure the appropriate diagnosis and treatment is selected for patients with hyperkeratosis.
Introduction
Hyperkeratosis refers to the increased thickness of the stratum corneum, the outer layer of the skin. Stratum corneum is composed of multiple layers of keratinocyte bodies that, during maturation, produced keratin and subsequently have lost their nucleus and cytoplasmic organelles. The result is a basketweave appearance of anucleate keratinocytes that protect the underlying cells during maturation.
Hyperkeratosis is subclassified as orthokeratotic or parakeratotic. Orthokeratotic hyperkeratosis refers to the thickening of the keratin layer with preserved keratinocyte maturation, while parakeratotic hyperkeratosis shows retained nuclei as a sign of delayed maturation of keratinocytes. Hyperkeratosis can be associated with dyskeratosis. It represents a premature (keratinocytes that are located below the granular cell layer) or abnormal keratinization of individual keratinocytes.
Hyperkeratosis, associated with other abnormalities in the skin biopsy, can be a key to the final histological diagnosis. Epidermal hypertrophy is a benign alteration of the skin that presents with acanthosis (increased thickness of the keratinocyte layers) and hyperkeratosis.
Etiology
Increased thickness of the stratum corneum can be due to several exogenous or endogenous processes and is related to increased production of keratin in the outer portion of the skin layer. In the majority of cases in clinical practice, it is mostly due to chronic physical or chemical damage such as friction or the use of aggressive soaps (especially those with a basic pH) but can also derive from chronic inflammation or a side-effect of different drugs, including chemotherapy.[1][2]
Hyperkeratosis happening in the context of a reactive state of the skin is the prototypical result of dermatitis. Another cause of hyperkeratosis is a nutritional deficit, especially in vitamin A deficiency, causing phrynoderma, where the skin can exhibit keratin plugs and hyperkeratosis of the hair follicles, atrophy, and squamous metaplasia of the sebaceous glands.[3][4][5]
Epidemiology
The incidence is unknown, as hyperkeratosis is the histopathologic aspect of many different pathologies, both benign and malignant.
Pathophysiology
The skin is composed of three layers: the epidermis, the dermis (composed of the superficial papillary and deeper reticular dermis), and the hypodermis. The skin has structural differences among the different areas of the body in terms of epidermal and dermal thickness, distribution of appendages, and pigmentation. The epidermis is composed of multiple layers of maturing keratinocytes: the basal layer (stratum basale), the squamous layer (stratum spinosum), the granular layer (stratum granulosum), and the cornified layer (stratum corneum). This stratified epithelium is in a constant process of self-renewing and exfoliation that takes 20-40 days to complete. The cells in the outer layer are the most differentiated in the keratinocyte line, composed almost entirely of keratin lamels of high molecular weight, and those are the ones that undergo desquamation, completing the maturation cycle.
When the epidermis is exposed to repetitive injury, it usually elicits an increased proliferative rate of the keratinocytes and accelerates their maturation. Keratinocytes also tend to produce more keratin, thus increasing the stratum corneum's thickness.
Injury to the epidermis, if acute, tends to result in edema, also called spongiosis. This is seen as a clear space between keratinocytes. Severe edema can cause the formation of intraepidermal vesicles as extracellular fluid accumulation between keratinocytes. If the process becomes chronic, the edema regresses, and the epidermis becomes hyperplastic as a response to the chronic insult. Epidermal hyperplasia is seen as a thickening of the epidermis and elongation of the rete ridges (called acanthosis), accompanied by ortho or para-hyperkeratosis. These are the typical transition phases of acute to chronic spongiotic dermatitis such as eczema.
Genetic mutations resulting in hyperkeratosis is seen in ichthyoses and keratoderma. There are several damages in keratin-encoding genes such as KRT1 and KRT10, which cause defects in keratin structure. Defective keratin causes irregular aggregates of intermediate filaments, which leads to cellular collapse and blistering. The barrier function is then compromised, and the skin reacts with compensatory hyperproliferation, which leads to hyperkeratosis.
Histopathology
Mechanical hyperkeratosis: This is characterized by increased keratinocyte activity mainly due to chronic pressure or friction on the skin. Lesions are benign demarcated hyperkeratotic lesions, mainly corn and callus. The presence of a central conical core distinguishes a corn from a callus. Corns are subtyped into hard corn (heloma durum) and soft corn (heloma molle). Both show dense hyperkeratotic stratum corneum with mild acanthosis, variable hypergranulosis, and collagenization of the superficial dermis with variable mucin deposition. There is usually no inflammatory infiltrate accompanying the lesion.
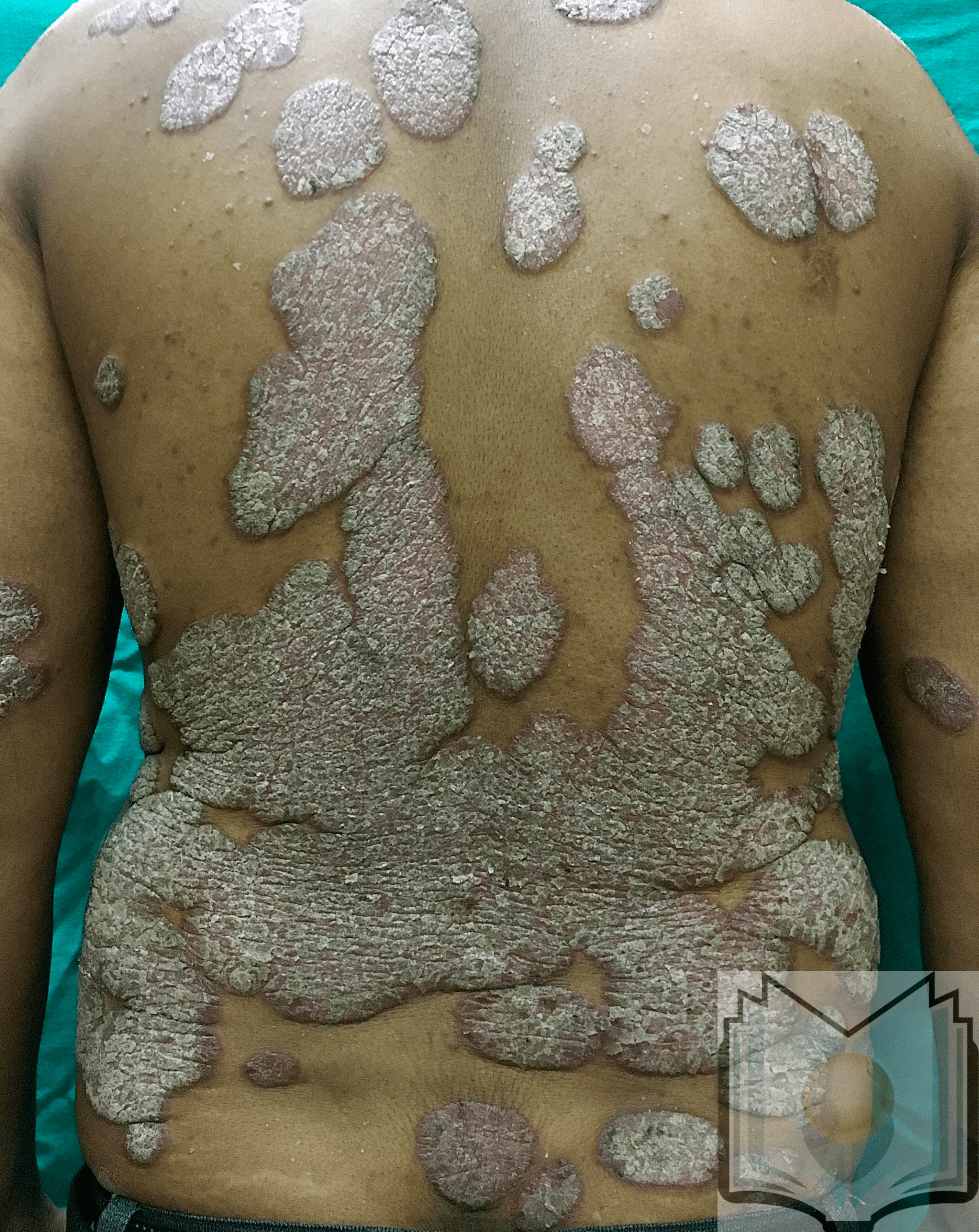
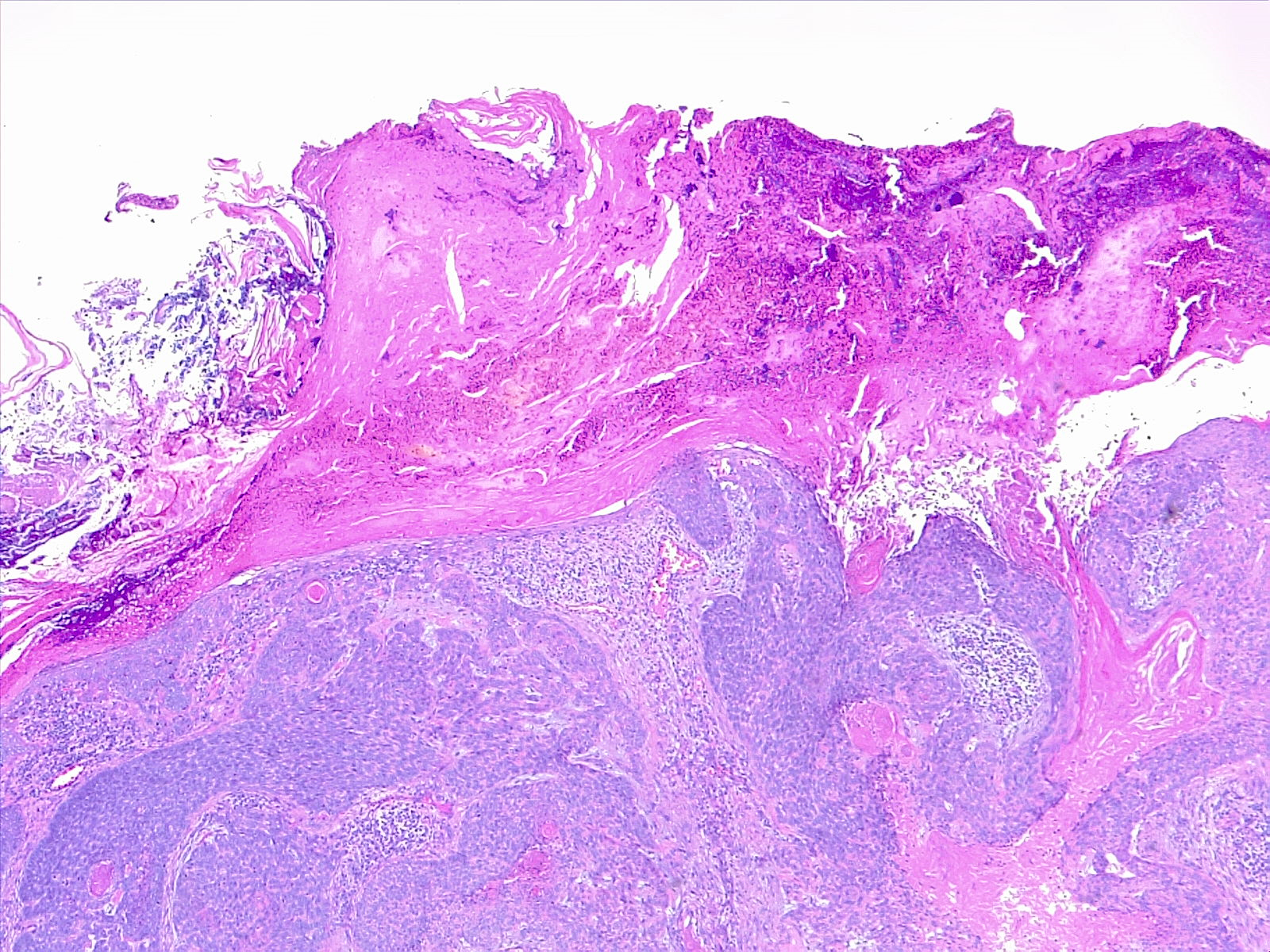
Psoriasis and psoriasiform dermatitis: It shows perivascular aggregates of lymphocytes in the dermal-epidermal junction with focal migration of leukocytes (neutrophils, lymphocytes) into the epidermis. There is increased epidermal proliferation and elongation of rete ridges giving an undulating appearance to the epidermis (papillomatosis) with or without spongiosis. The altered differentiation of keratinocytes results in hyperkeratosis with parakeratosis. Psoriasis also shows the formation of microabscesses by small aggregates of neutrophils in the upper epidermis (pustules) or in the stratum corneum (Munro microabscesses).
Interface and lichenoid dermatitis: Dense aggregates of lymphocytes along the dermal-epidermal junction associated with vacuolation of basal keratinocytes.[6] There is dyskeratosis, hyperkeratosis, and is sometimes associated with hypergranulosis.
Verrucae vulgaris and plana are characterized by marked hyperkeratosis, papillomatosis, and acanthosis. A typical feature is the presence of koilocytes, cells infected with papillomavirus which have structural changes like perinuclear halos and keratohyalin granules. Koilocytes can be absent in older lesions, but when present, are located in the upper stratum spinosum or granulosum. Parakeratosis may be present.
Seborrheic keratosis features marked hyperkeratosis, papillomatosis, and acanthosis. Pseudo-cysts and horn cysts are frequently present. There may be lymphocytic infiltrate and pigmentation as secondary features when irritated or inflamed.
The ichthyoses are a group of diseases caused by altered keratinization. The most common forms are ichthyosis vulgaris, X-linked, congenital, and epidermolytic hyperkeratosis.[7] They can be hereditary or acquired during life.[8][9] All of the forms show a defective epidermal barrier that induces hyperkeratosis, skin scaling, and inflammation.
Squamous cell carcinoma (SCC) is a neoplastic proliferation of atypical keratinocytes, restricted only to the epidermis (SCC in situ or Bowen's disease) or infiltrating the dermis (infiltrative SCC). Classic features are hyper-parakeratosis and loss of the granular layer.
Toxicokinetics
As previously said, hyperkeratosis can be the result of different pharmaceutical compounds mainly used for oncologic treatments. Hyperkeratotic drug reaction occurs commonly with tyrosine kinase inhibitors, cytotoxic chemotherapeutic agents, and immunomodulators or immune checkpoint inhibitors.[10][11][12][13] The disruption of epidermal homeostasis alters the keratinocyte differentiation process or even interacts with the keratinocyte survival mechanism. Commonly described types of reactions include hand-foot hyperkeratosis, palmoplantar keratoderma, psoriasis, keratosis pilaris, pityriasis rubra pilaris, Grover's disease, and contact dermatitis with hyperkeratosis.[1]
BCR-ABL inhibitors (mainly nilotinib and dasatinib) are commonly used for ontological target therapy, and the cutaneous side effects are only second to the hematologic sequelae. They are usually transitory and not severe. The most common dermatological side effect is a pruritic skin rash, while chronic dermatological side effects include psoriasis, lichenoid hyperkeratosis, pityriasis, and others.[14][15][16]
Multikinase-inhibitors (VEGF, PDGFR, EGFR, KIT, RET, Flt3, and RAF) affect the skin homeostasis and give rise to many different cutaneous manifestations, mainly with hyperkeratosis in the form of hyperkeratotic hand-foot skin reaction.[14] Hyperkeratosis occurs in the sites of friction or pressure, mainly soles, causing pain and limitation of the daily activities.[17][18]
Inhibition of the RAF pathway can lead to paradoxical MAPK activation and induce dimerization of RAF isomers. Keratinocyte proliferation is characteristic of B-RAF inhibitors, causing a broad spectrum of epidermal toxicities ranging from a verrucal reaction to squamous cell carcinoma. The use of the RAF-pathway inhibitor induces the rapid appearance of keratinocyte malignancies, mainly squamous cell carcinomas of the skin, even within the first week after the starting them.[19] Those compounds, instead of actually inducing skin cancers, can reveal and facilitate the genetic events in the oncogenesis of keratinocytes. Characteristically, the verrucous lesions lack viral infection and the cytopathic viral changes seen in histology, and the keratinocyte appears minimally to mildly atypical. The epidermis shows only acanthosis and hyperkeratosis. Experimental treatment with downstream inhibition of the MAPK pathway (MEK-RAF blockade) greatly reduces the impact of this lesion during treatment.[20]
The dermo-epidermal changes related to tattoo procedures can elicit skin reactions with hyperkeratosis patterns.[21][22][23] The pathogenesis is not clear, and etiology is probably multifactorial involving the local trauma, inflammatory reaction, ink composition, UV exposure, and genetic predisposition. [24][23] Of all dyes used for tattooing procedures, red dyes cause allergic contact dermatitis and characteristic patterns of plaques, hyperkeratosis, ulcerating reactions, urticaria, and skin rashes.
History and Physical
Hyperkeratosis is a histopathological term defining a thickened stratum corneum and may be present in many different skin conditions, with many possible overlaps. History and clinical evaluation are key, and the main goal is to collect as much information as possible and discern which cases require a histopathological diagnosis to direct the most appropriate treatment.
The history comprises the age of the patient, family history, exposure to toxic substances, drugs, occupational duties, anamnesis of the current lesion, concomitant pathologies, and treatments. In those patients where the diagnosis was already established, it is appropriate to reevaluate it, monitor progression and complications following the treatment.
The physical examination must be thorough to exactly understand the extent of the disease. Except for localized disease, it is important to inspect the entire skin surface, including scalp, eyelids, ears, perineum and genital mucosa, hair, and nails. The lesion should be described in terms of color, texture, shape, and distribution. Surrounding skin should be examined as well to detect the presence of generalized xerosis (dryness), seborrhea, hyper or hypohidrosis (sweating), texture, photoaging such as lentigines, actinic purpura, rhytides.
Small folliculocentric keratotic nodules can be found in cases of keratosis pilaris, where papules are centered on small hair follicles, and it can be associated with erythema. On close examination, it is possible to recognize a small coiled hair beneath the papule formed by a keratin plug.
Scaling is an important finding in cases of hyperkeratosis. Scales may be described as soft, rough, greyish, bran-like, and so on. Crusts should not be confused with scale as it is the result of dried fluid on the epidermis (serum, blood, pus, or a combination of those) and not thickening of the epidermis. Lichenification is a thickening of the skin and results from chronic injuries such as repetitive scratching. It is present in most chronic eczematous or neurogenic processes.
Evaluation
Dermoscopy is noninvasive and allows visualization of the skin structures in the epidermis, dermo-epidermal junction, and superficial dermis.
A biopsy is essential in cases in which the clinical setting is overlapping with different entities having distinctive histopathologic findings. For an ideal full-thickness biopsy, it is important to include the hypodermis. This can be performed with a simple 3 mm punch that minimizes scarring in the affected area. Any smaller size is at risk of being non-diagnostic.
Patch tests can be useful for identifying the causative allergen if an allergic dermatitis is suspected. Clinical clues are the presence of persistent, pruritic, eczematous eruptions in which any other identifiable cause has been excluded. If the patient tests positive, they should be encouraged to avoid the specific allergen. A follow-up after a few weeks of allergen avoidance is strongly recommended.
Treatment / Management
Basic skincare measures are important to prevent excessive dryness and to encourage exfoliation. Those remedies include soaps with skin-specific pH, soap-free cleansers, and avoidance of hot baths. Emollients and topical keratolytic agents (lactic acid, salicylic acid, urea) should be advised to be applied over affected areas at the appropriate times.
Sharp debridement is helpful in benign hyperkeratoses such as callus and corns to reduce the pressure and the amount of hyperkeratotic tissue. A chisel blade may be used to remove the keratin plug, providing relief to the area.
Surgical procedures have limited relevance in the treatment of hyperkeratosis. In cases of untreatable plantar keratosis with significant daily limitation, skin grafts with rotation skin flap have been demonstrated effective.[25][26]
Corticosteroids are the treatment of choice for inflammation-driven diseases such as lichen planus or psoriasis. Topical application is the best choice for localized disease. Topical applications should last one to two weeks.
Immunosuppressant or immunomodulators (cyclosporin, hydroxychloroquine, mycophenolate mofetil, sulfasalazine, alefacept, efalizumab) can be used in severe recurrent cases.
Topical calcineurin inhibitors (tacrolimus or pimecrolimus) can also be used.
Retinoids, topical or oral-based, are used in disorders of keratinization such as ichthyoses, keratosis folliculitis, and psoriasis. Topical administration is variable and must be evaluated in the appropriate clinical context; treatment usually lasts 8 to 12 weeks.
Combination treatments with lasers (e.g., pulsed-dye laser, 755-nm alexandrite laser, 810-nm diode laser, 1064-nm Nd:YAG laser) and microdermabrasion are noninvasive techniques currently under approval for different hyperkeratotic diseases.
Differential Diagnosis
The overall differential diagnosis for hyperkeratosis is extremely broad and includes benign dermatological diseases as well as malignant ones. Overlapping clinical features make the diagnosis and workup challenging. A correct anamnesis, patient examination, and the appropriate use of skin biopsy are useful for the differential diagnosis.
Differential diagnosis of hyperkeratosis:
- Callus and Corns
- Keratosis plantare
- Chronic folliculitis
- Atopic dermatitis
- Psoriasis and psoriasiform dermatitis
- Lichen planus and lichenoid dermatitis
- Keratosis pilaris
- Ichthyoses
- Seborrheic keratosis
- Actinic keratosis
- Keratoacanthoma
- Paraneoplastic syndromes
- Squamous cell carcinoma
- Basal cell carcinoma
Prognosis
The prognosis is related to the specific dermatological disease that is causing hyperkeratosis. Hyperkeratosis usually improves with treatment, but some cases may become persistent.
Complications
Psychosocial distress can be elicited by the cosmetic appearance of hyperkeratosis, especially in exposed areas such as the face, scalp, and neck. Another complication that can occur is scarring secondary to the traumatic manipulation of the lesions by the patient. Clinicians must educate patients on the necessity of using the prescribed topical medications and refrain from manipulating the lesions.
Consultations
Dermatological consultation should be always considered in difficult cases or when therapy is not satisfactory. Pathological consultation should also be considered when skin biopsy can help in the diagnosis.
Deterrence and Patient Education
Patients should be educated that basic skincare measures such as hygiene, hydration, exfoliation are important to maintain functional skin even in the affected areas. Instructions for the other precautions should be given based on the specific dermatological diagnosis.
Enhancing Healthcare Team Outcomes
Interaction with the patient and healthcare personnel involved in clinical care is essential for a correct diagnosis and follow-up. Clinicians should inform patients about the diagnosis and expectations of treatment success. Depending on the final diagnosis, the treatment may require time and can be frustrating if it requires multiple months and repetitive follow-up reviews in the clinic. The clinician is encouraged to use a stepwise approach to hyperkeratosis.
The initial evaluation must be very accurate, and a skin biopsy should be performed in the correct setting, and treatment can be started as soon as the diagnosis is clear to avoid mistreatment and disease exacerbation. Initial treatment usually consists of emollients, topical keratolytics, and re-evaluation. If there is no response to a topical trial, the clinical setting should be re-evaluated, and the patient must always be informed about other treatment options. Depending on the diagnosis, therapy can include corticosteroids, retinoids, topical anti-inflammatories, vitamin-D derivatives, phototherapy, and laser treatment. It is important to always consider the possibility of a dermatology referral for those that do not respond to treatment.
The nursing personnel has a significant role in counseling and monitoring patient progression. Pharmacists are frequently involved in patient care being sometimes the first to evaluate the lesion before medical referral. During follow-up, the pharmacist can be a helpful reference for the patient and may be asked for counseling or for administration instructions. An interprofessional team approach is encouraged for optimal patient results.