Continuing Education Activity
Split-thickness skin grafts are versatile adjuncts to wound closure in burns, trauma, reconstruction, and other large wounds. This activity outlines and explains the role of the healthcare team in managing patients who undergo split-thickness skin grafting for various etiologies.
Objectives:
- Identify the phases of healing, indications, and contraindications of split-thickness skin grafts.
- Describe the equipment, preparation, and technique involved in split-thickness skin grafting.
- Review the utility of various types of split-thickness skin grafts and their place within the reconstructive ladder.
- Summarize the potential complications of split-thickness skin grafts and summarize interprofessional team strategies for improving care to optimize outcomes of split-thickness skin grafts.
Introduction
Skin grafting is the transfer of cutaneous tissue from one portion of the body to another, often used to cover large wounds. The rationale of skin grafts is to take skin from a donor site that will heal and transfer the skin to an area of need. After incorporation, skin grafts provide wounds with protection from the environment, pathogens, temperature, and excessive water loss like normal skin.
A split-thickness skin graft (STSG), by definition, refers to a graft that contains the epidermis and a portion of the dermis, which is in contrast to a full-thickness skin graft (FTSG) which consists of the epidermis and entire dermis. Unlike flaps, skin grafts do not have their own blood supply, so they must rely on a well-vascularized wound bed for graft in-growth. Split-thickness skin grafts are obtainable from multiple sources (autograft, homograft, allograft, or xenograft), multiple anatomical locations, and in various thicknesses. Most commonly, STSG autografts are taken from the lateral thigh, as well as trunk, as these sites are both aesthetically hidden, as well as easy to harvest from due to their broad surfaces. Split-thickness skin grafts classify according to their thickness into thin STSGs (0.15 to 0.3mm), intermediate STSGs (0.3 to 0.45mm), and thick STSGs (0.45 to 0.6mm).[1][2] Because split-thickness skin graft donor sites retain portions of the dermis, including dermal appendages, the donor site can regrow new skin in 2 to 3 weeks. Thus, donor sites can be used more than once after appropriate healing has taken place, which makes STSGs versatile in burn surgery and large wounds where there are limited donor sites.
The advantages and disadvantages of STSGs are best highlighted by comparison with FTSGs. Considerations of proper skin graft selection should include graft take, contracture of skin graft, donor site morbidity, aesthetic match, and durability.
- Graft Take: The thicker a skin graft, the more metabolically active it is, and the worse is it's nutrient diffusion. FTSGs and thick STSG's require more robust recipient wound beds than thin STSGs. Thick grafts should be avoided in unhealthy wound beds such as chronic ulcers.
- Contracture: All skin grafts undergo primary and secondary contractures. Primary contracture is the immediate reduction in the size of skin graft after it has been harvested, caused by passive recoil of elastin fibers in the dermis. As FTSGs have a greater amount of dermis, primary contracture is more significant in FTSG than STSG. Secondary contracture is the shrinkage of the skin graft in the wound bed over time, caused by myofibroblasts. Secondary contracture is greater for STSGs than FTSGs, as the additional dermis in FTSGs is resistant to the pull of myofibroblasts. Clinically, STSG placement should not be in aesthetically sensitive areas that could become deformed with contractures such as around the eyelids, face, and mouth.
- Donor Site Morbidity: The multipotent stem cells responsible for STSG donor site reepithelialization primarily reside in the hair follicles. By preserving portions of the dermis and thereby hair follicles, STSG donor sites regrow new skin and are reusable. Thin STSGs have the least donor site morbidity and regrow new skin the fastest. Full-thickness skin grafts involve excision of the entire thickness of skin, and thus adnexal structures, necessitating primary closure.
- Aesthetic Match: Skin grafts should ideally match the recipient bed in color, texture, and overall appearance. Full-thickness skin grafts commonly provide an appropriate color match, whereas STSGs are more likely to be hypo/hyperpigmented. Additionally, the meshing of STSGs significantly alters the aesthetics of STSGs.
- Durability: As the dermis provides strength and viscoelastic properties to the skin, the consideration of dermal thickness is essential for each specific wound. For example, thick STSGs or FTSG are common choices to cover mechanically demanding areas of the body, including the palms, soles, and joints, whereas thin STSGs do not withstand such forces as well.[3]
Disadvantages of STSGs compared to other reconstructive techniques include at times poor resemblance to surrounding recipient site skin (color match and texture if meshed), high susceptibility to trauma, poor sensation of the recipient site, need for anesthesia/surgery (compared to secondary intention healing), and prolonged need for wound care of both the donor and recipient sites (compared to flap closure).
Anatomy and Physiology
Split-thickness skin grafts contain the epidermis and a portion of the dermis. The epidermis is the outermost layer of skin, comprised primarily of keratinocytes. The epidermis is a thin, semitransparent layer that provides a significant barrier function. The epidermis also includes melanocytes, Langerhans cells, Merkel cells, and nerve endings. Skin adnexal structures, including hair follicles, sweat glands, and sebaceous glands, are epidermal derivatives that invaginate into the dermis. Stem cells from the adnexal structures, specifically hair follicles, are responsible for the reepithelialization of skin graft donor sites. The dermis is the fibrous layer below the epidermis composed of collagen, glycosaminoglycans, and elastin. The upper portion of the dermis, the papillary dermis, contains plexi of blood vessels and nerves. These plexi provide nutrients to the epidermis via diffusion. The undulating surface between the epidermis and papillary dermis portends stability between the two layers. The deeper portion of the dermis, the reticular dermis, contains robust collagen fibers. The dermis provides strength and stability to STSGs.
As mentioned above, STSGs do not have their own blood supply, so they must rely on the underlying wound bed for nutrients and blood supply. Presuming a stable, healthy, and well-vascularized wound bed, skin grafts take occurs in three commonly described steps:
- Imbibition:
- The skin graft passively absorbs oxygen and nutrients from the wound bed. During this phase, the skin graft is ischemic and survives on diffusion alone until reestablishing graft vasculature. The graft is pale/white during this time. Split-thickness skin grafts can tolerate up to 4 days of ischemia.[4][5][6]
- Inosculation:
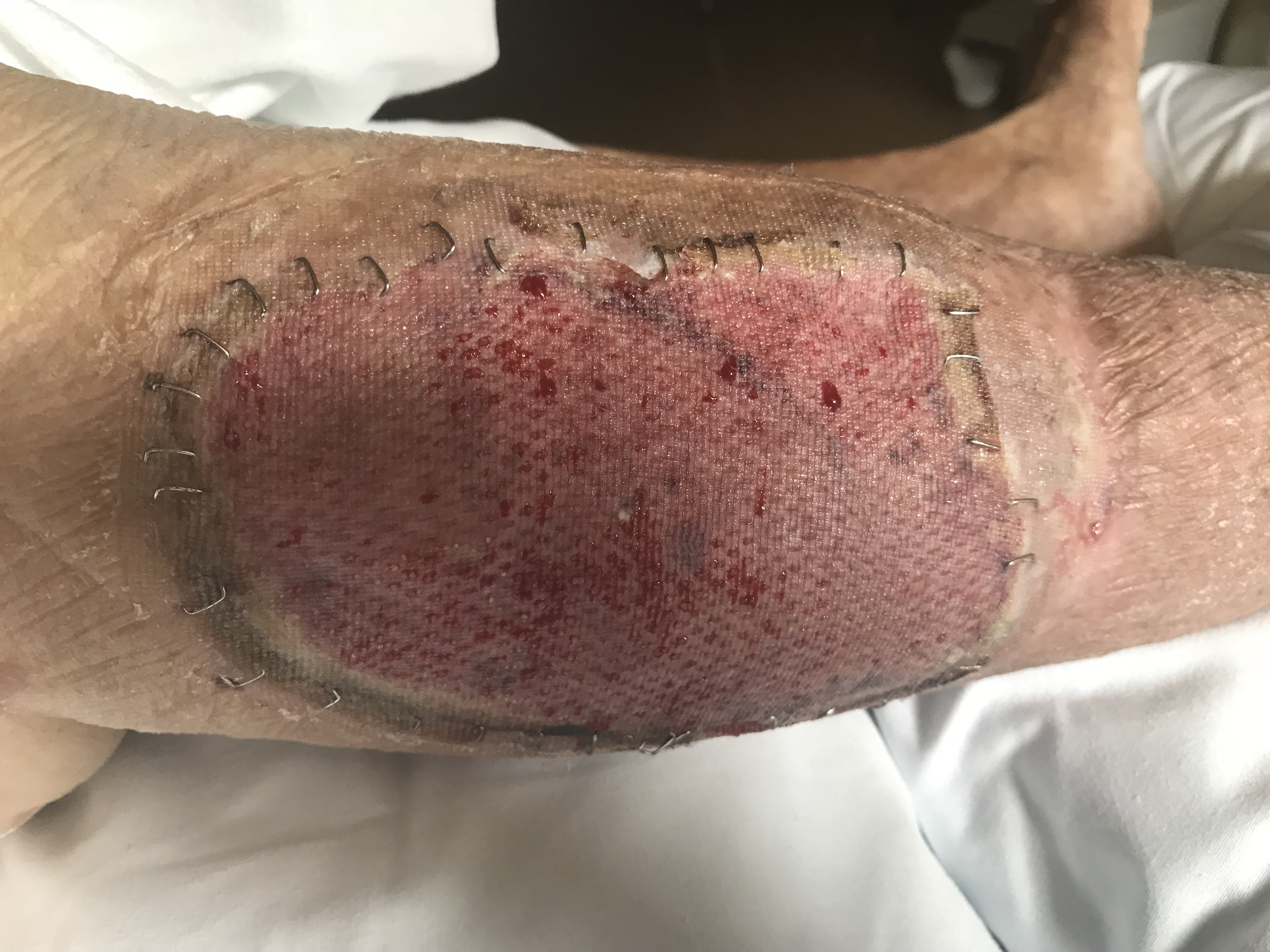
- A vascular network is established between the cut vessels on the underside of the skin graft and the capillary beds in the wound bed, establishing a vascular connection. The graft becomes pink at this point. Inosculation typically occurs at around 48 hours after graft placement.[7]
- Revascularization
- Several hypotheses exist regarding the exact mechanism of revascularization. The neovascularization theory is of new vessel ingrowth into the graft from the recipient wound bed. The endothelial cell ingrowth theory suggests that endothelial cells proliferate and slide from the recipient site by following pre-existing vascular basal lamina as structure, with graft endothelial cells eventually degrading.[8][9][10][11]
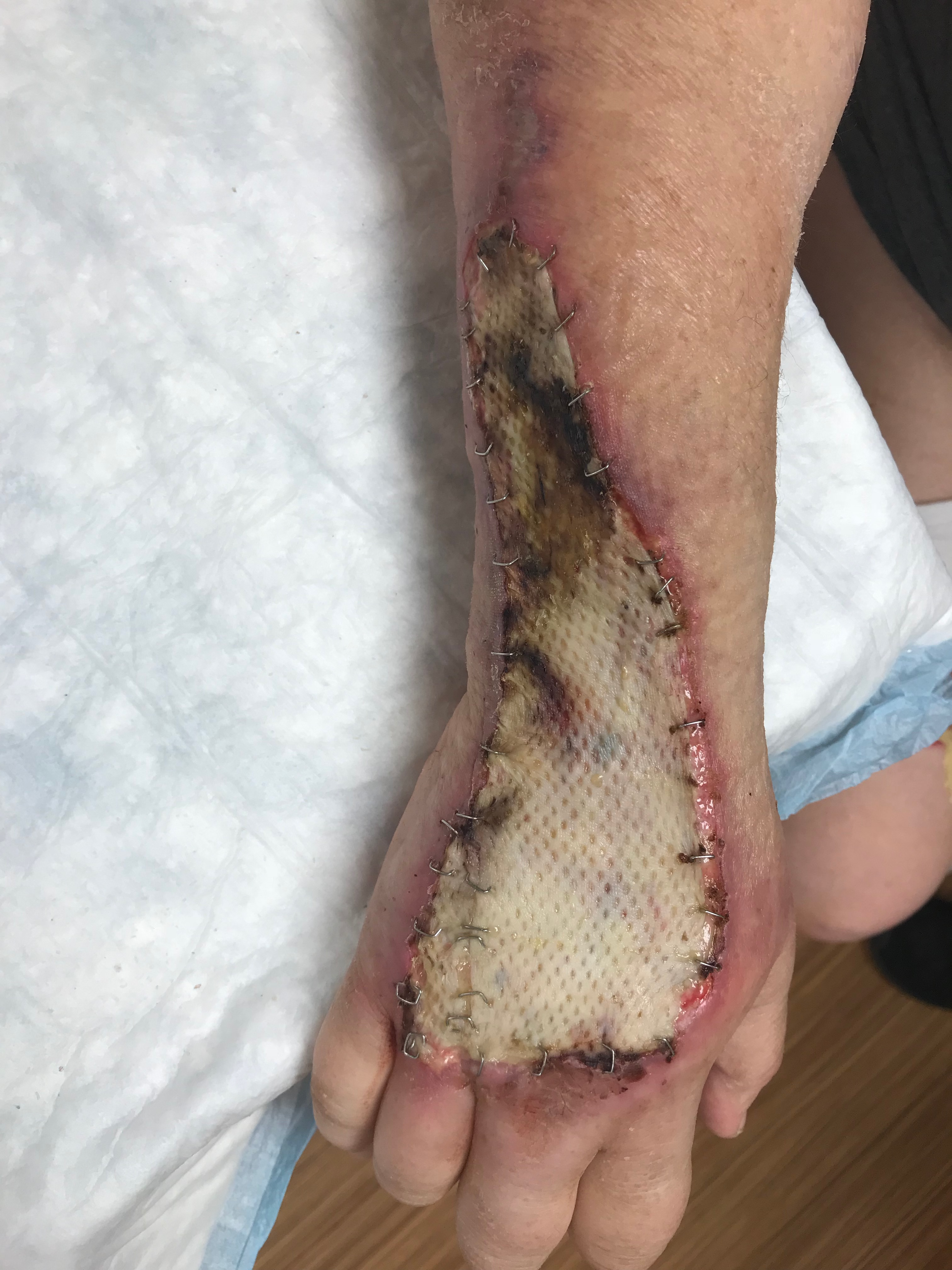
Clinically, skin grafts are secured into place and often bolstered until postoperative day 5 to 7 to allow the skin graft to go through the above steps, ensuring the best skin graft take. Split-thickness skin grafts are typically adherent after 5 to 7 days upon completion of the stages of wound healing. Once the graft has integrated into the wound bed, it undergoes a maturation process that takes over one year to complete. Skin graft maturation can last up to several years in burn patients. The maturation process includes changes in pigmentation, flattening, and softening. Even after maturation, meshed split-thickness skin grafts may maintain a cobblestone appearance.
Split-thickness skin grafts can be meshed to increase the overall size of the graft, which is useful in cases where the wound size is greater than the available donor site. In a meshed graft, the bridges of meshed skin follow the above phases of skin graft take — the spaces between the skin heal via epithelialization from the skin bridges. Meshing can occur in various ratios such as 3/8 to 1, 1 to 1, 2 to 1, 3 to 1, and even 6 to 1. The greater the ratio, the larger the spaces between the skin bridges, and the more epithelialization necessary to heal the space in-between. Meshing a skin graft effectively expands the skin graft to increase the area that can be covered by the skin graft. Additionally, the holes between skin bridges act as drainage holes to prevent fluid, blood, or seroma build-up between the recipient wound bed and skin graft, which would cause graft failure.
Indications
Surgeons should assess each wound individually and utilize the reconstructive ladder to find a wound closure solution that is ideally the simplest, the fastest, and with the best aesthetic outcome.
Split-thickness skin grafts play an integral part of the reconstructive ladder. They are indicated when simpler methods of wound closure will not suffice, such as healing by secondary intention, primary closure, or negative pressure wound therapy.[12] A prerequisite of skin grafting includes available donor sites and recipient sites that are well-vascularized and clean. Typically skin grafts are used to cover deep partial-thickness skin defects, full-thickness skin defects, or placement over muscle; however, they can survive on any wound bed with vascularity including tendon with intact paratenon (forearm, hand, fingers), cartilage with intact perichondrium (ears), bone with intact periosteum (skull), and even vascularized biologic dressings. If these thin vascular layers are not in place, STSGs will fail.
Split-thickness skin grafts are otherwise indicated in acute skin loss (burn wounds, traumatic wounds, infection), chronic skin loss (leg ulcers), and as adjuncts to other procedures (to cover a muscle flap).
Contraindications
Absolute contraindications: wounds with an active infection, active bleeding, or known cancer. Wounds with exposed bone, tendon, nerve, or blood vessel without appropriate vascular layer.
Relative contraindications: wounds over joints or key anatomic landmarks in which contraction would reduce mobility and/or aesthetics (i.e., wrist, elbow, eyelid), and previously irradiated wounds.
Clinicians should consider patient factors such as tobacco use, anti-coagulant use, bleeding disorder, chronic steroid use, or malnutrition on a case-by-case basis.
Equipment
Skin Graft Harvest
Split-thickness skin grafts are harvestable in several ways, including with a surgical knife, oscillating Goulian knife, and most commonly with an air or electric powered dermatome. Manually harvesting a uniform depth skin graft is challenging and may result in irregularities in the donor site as well as a skin graft. Thus, powered dermatomes are a frequent choice as they offer harvest consistency, as well as adjustability in graft thickness (on the order of thousandths per inch) and width (1-inch, 2-inch, 3-inch, and 4-inch guards).
Skin Graft Meshing
Meshing methodologies vary from manual perforations in the skin graft with a surgical scalp to a hand-powered meshing device (mesher). Surgeons frequently utilize meshing devices as they apply multiple slits at regular intervals and in preset ratios. Commonly used ratios include 3/8 to 1, 1 to 1, 2 to 1, 3 to 1, and even 6 to 1. The split-thickness skin graft gets placed into the mesher and hand-cranked through the machine. Meshing a skin graft allows the graft to stretch, increasing the area that can be covered by the skin graft. The higher the meshing ratio, the further a skin graft can stretch; however, the longer it will take for the skin graft to heal completely due to the increased area to epithelialize. The holes between skin bridges act as drainage holes to prevent fluid, blood, or seroma build-up between the recipient wound bed and skin graft, which would cause graft failure.
Other Equipment
Mineral oil is used to lubricate the donor site before skin graft harvest and allows for improved sliding of the dermatome. An epinephrine soaked sponge (1 vial of 1:1000 epinephrine in 500 milliliters of 0.9% normal saline) is placed on the donor site following harvest to minimize blood loss. Surgical pick-ups (Adson tissue forceps) are used to retrieve the skin graft from the dermatome.
Securing the Skin Graft
The STSG gets secured to the recipient site with skin staples or a running simple interrupted suture. The application of skin staples is must faster than suture; however, it does require eventual removal of staples once the skin graft has healed. Skin staples are applied at regular intervals around the extent of the skin graft. Typically dissolvable suture like chromic is utilized for securing the skin graft as it dissolves around the same time the skin graft becomes adherent and does not require removal. Securing split-thickness skin grafts with fast-clotting fibrin glue with high-concentration fibrin has also been reported.[13]
Dressings
A tie over bolster of petroleum-infused gauze, cotton balls, and non-dissolvable suture is frequently placed on smaller STSG recipient sites. A negative pressure wound vacuum is another viable option for areas that are difficult to bolster. In cases of large volume STSGs, petroleum-infused gauze and bulky gauze/kerlix are placed over the grafts. The grafts should not be left open to the air. The STSG donor site gets covered with petroleum-infused gauze and clear adhesive, followed by several layers of kerlix and an ACE wrap. Alternatively, the donor site can be covered with an anti-microbial foam dressing, kerlix, and ACE wrap.
Personnel
A surgeon and an assistant can harvest split-thickness skin grafts.
Preparation
Consent
Part of the preparation process includes informed patient consent. The surgeon should discuss the postoperative course expected timeframe for donor and recipient site healing and the concept of skin grafting with the patient.
Wound Bed
Aside from preparing the equipment mentioned above, the most crucial aspect of preparation is creating a clean wound bed suitable to accept a split-thickness skin graft. Debridement of the wound bed is possible in multiple ways. The recipient bed should be debrided with a scalpel, Norsen debrider, dermatome, or hydrosurgery device until the wound bed has healthy bleeding tissue at the base.[14] Freshen up the edges as necessary. The wound edges and base must be free of nonviable tissue, purulence, and exudate, such that all aspects of the wound should have pinpoint bleeding from the margins.
Without a clean wound base, the split-thickness skin graft cannot undergo the normal phases of skin graft healing.
Donor Site
The donor site should be chosen based on the amount of skin graft needed, surgical positioning of the patient, ease of donor site harvest, and aesthetics. Broad, flat regions like the anterolateral thighs, back, trunk, lateral arm/forearm, lateral lower leg serve as the easiest donor sites when using a mechanical dermatome because they are firm surfaces against which the dermatome operator can push. The thighs and back provide a large surface area from which to harvest a skin graft. Aesthetically, donor sites that will be routinely covered by clothing are typically chosen, such as the thighs. Skin from the back and the thighs is typically thicker than skin from other parts of the body; thus, skin graft harvest thickness requires adjustment for this (thicker graft used in the area of high stress, thinner graft used to match thin recipient skin). In large wounds or burns, donor sites are subject to limitation by the location of remaining healthy skin.
Technique or Treatment
The technique for harvesting a split-thickness skin graft with an air-powered dermatome will be described as it is a common harvesting method.
Preparation of Wound Bed and Split-thickness Skin Graft Harvest
- Debride the recipient bed with a scalpel, Norsen debrider, dermatome, or hydrosurgery device until the wound bed has healthy bleeding tissue at the base. Freshen up the edges as necessary.[14]
- Measure the recipient's wound bed. These measurements will equate to the size of the skin graft harvested.
- Connect the dermatome to the air-source. Temporarily turn the dermatome on to ensure appropriate power, indicated by a high pitched hum.
- Apply a fresh dermatome blade into the dermatome and choose the desired guard plate width. Widths span 1-inch to 4-inches. Once assembled, select the thickness of the split-thickness skin graft by turning the dial on the side of the dermatome.
- Based on the width of the guard, calculate the length of the graft to be harvested based on the total size of the skin graft desired. Mark the length on the donor site with a surgical marker.
- Apply mineral oil to the dermatome and donor site to optimize gliding.
- In conjunction with your assistant, use a towel or towel clamps to pull the donor site in opposite directions parallel to the path of the dermatome, making the donor site taught.
- Turn the dermatome on and bring it into contact with the skin at a 45-degree angle. After making skin contact, flatten the dermatome to be nearly parallel with the skin.
- Apply firm downward pressure as the dermatome smoothly pushes forward.
- Upon achieving the desired length of the graft, angle the dermatome upwards at a 45-degree angle off of the skin.
- Turn the dermatome off and a use surgical scalpel or scissors to cut the skin graft from the donor site.
- Pull the skin graft from the dermatome using tissue forceps.
- Place the split-thickness skin graft in normal saline until it is to be used.
- Apply an epinephrine-soaked to the donor site to reduce blood loss.
Meshing and Securing the Graft
- Mesh the split-thickness skin graft if desired. The surgeon can perform this process with a scalpel (fenestrating or "pie-crusting") or a skin graft mesher. If using a mesher, spread the skin graft out before entrance into the mesher to ensure appropriately spaced slits. Gently guide the skin graft out of the mesher
- Carefully transfer the skin graft to the donor site, placing the graft onto the recipient bed dermis side down. If the skin graft is placed epidermis side down, the skin graft will fail. Typically the dermis is a lighter shade than the epidermis; however, it can be challenging to differentiate on lighter shade skin. The skin graft will always curl down toward the dermis, so use this to orient the graft correctly.
- Secure the graft with skin staples or suture.
- Apply a tie-over bolster; negative pressure wound vacuum, or dressings as indicated by the recipient site.
- Dress the donor site, as indicated above.
Complications
Short term: Any buildup of fluid between the split-thickness skin graft and wound bed will jeopardize skin graft take, including seroma, hematoma, and infection. Shear or traction injury also disrupts skin graft healing. The graft can have incomplete (less than 100%) take or complete nontake.
Long term: Wound contracture and aesthetic issues, including pigmentary and texture differences between the skin-graft and donor site, are common.
Skin graft take: Split-thickness skin graft take is consistently reported at around 70 to 90%, even when accounting for a variety of recipient wound beds.[15][16][17][18] TBSA burned over 35%, age greater than 55 years old, and the presence of diabetes mellitus can adversely affect the success rate of STSGs.[16]
Clinical Significance
Split-thickness skin grafts typically become adherent to the recipient wound bed 5 to 7 days following skin graft placement. The dressings placed intraoperatively are kept in place until 5 to 7 days postoperatively to minimize shear and traction to the healing skin graft. At 5 to 7 days postoperatively, the dressings are taken down, and the skin graft inspected. The graft should be pink at this point, indicating successful inosculation and revascularisation. For the next 7 to 14 days, dressing changes should be performed every 24 to 72 hours. These dressing changes typically consist of petroleum-infused gauze, bulky gauze/kerlix, ACE wrap, or wound VAC, and can be performed by the patient, home nursing care, or wound clinic. At the 2 to 3 week postoperative mark, the skin grafts should be adherent and epithelialized so the patient may resume showering and bathing, and may stop frequent dressing changes. Lotion can be applied to the skin graft to promote continued healing.
Enhancing Healthcare Team Outcomes
These types of skin grafts require a collaborating interprofessional team.
The donor sites, recipient sites, and date of next dressing changes should be delineated in medical notation by the surgical team so the entire healthcare team can treat the patient appropriately. In this way, recently grafted wounds are not disturbed, and graft take is not jeopardized. Burn patients should ideally receive care for in a burn center with experienced nurses, techs, social workers, and therapists. Nurses should be fully aware of the post-operative management of patients with skin grafts. The wounds should be monitored closely for bleeding, infection, and ischemia. Any change in the wound requires immediate communication with the surgeon. Additionally, since many of these patients remain immobile, nurses should ensure they are on deep vein thrombosis. Wound dressing changes should take place according to the preference of the surgeon. Once the wounds have healed, some patients may require physical therapy. Others may need to wear compression garments to prevent hypertrophic scarring. A wound care nurse should monitor these patients until the healing is complete and report any concerns to the surgeon for evaluation. The success of skin grafts depends not only on the surgeon but the interprofessional team looking after the patient. [Level 5]