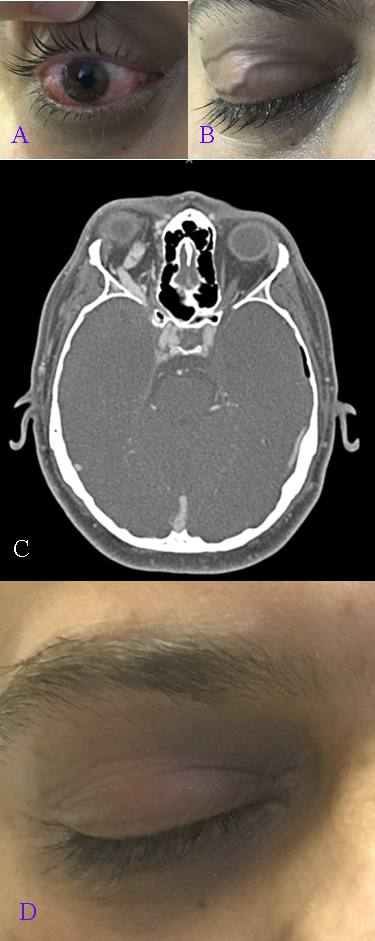
[1]
Ellis JA, Goldstein H, Connolly ES Jr, Meyers PM. Carotid-cavernous fistulas. Neurosurgical focus. 2012 May:32(5):E9. doi: 10.3171/2012.2.FOCUS1223. Epub
[PubMed PMID: 22537135]
[2]
Barrow DL,Spector RH,Braun IF,Landman JA,Tindall SC,Tindall GT, Classification and treatment of spontaneous carotid-cavernous sinus fistulas. Journal of neurosurgery. 1985 Feb
[PubMed PMID: 3968564]
[3]
Henderson AD,Miller NR, Carotid-cavernous fistula: current concepts in aetiology, investigation, and management. Eye (London, England). 2018 Feb
[PubMed PMID: 29099499]
[4]
Tripathy K,Sharma YR,Chawla R,Basu K,Vohra R,Venkatesh P, Triads in Ophthalmology: A Comprehensive Review. Seminars in ophthalmology. 2017
[PubMed PMID: 26148300]
[5]
Williams ZR, Carotid-Cavernous Fistulae: A Review of Clinical Presentation, Therapeutic Options, and Visual Prognosis. International ophthalmology clinics. 2018 Spring
[PubMed PMID: 29517654]
[6]
Golnik KC,Miller NR, Diagnosis of cavernous sinus arteriovenous fistula by measurement of ocular pulse amplitude. Ophthalmology. 1992 Jul
[PubMed PMID: 1495796]
[7]
Kai Y,Hamada J,Morioka M,Yano S,Kuratsu J, Treatment of cavernous sinus dural arteriovenous fistulae by external manual carotid compression. Neurosurgery. 2007 Feb
[PubMed PMID: 17290175]
[8]
Park SH,Park KS,Kang DH,Hwang JH,Hwang SK, Stereotactic Radiosurgery for Dural Carotid Cavernous Sinus Fistulas. World neurosurgery. 2017 Oct
[PubMed PMID: 28465265]
[9]
Gemmete JJ,Ansari SA,Gandhi DM, Endovascular techniques for treatment of carotid-cavernous fistula. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2009 Mar
[PubMed PMID: 19458580]
[10]
Miller NR,Monsein LH,Debrun GM,Tamargo RJ,Nauta HJ, Treatment of carotid-cavernous sinus fistulas using a superior ophthalmic vein approach. Journal of neurosurgery. 1995 Nov
[PubMed PMID: 7472552]
[11]
Preechawat P,Narmkerd P,Jiarakongmun P,Poonyathalang A,Pongpech SM, Dural carotid cavernous sinus fistula: ocular characteristics, endovascular management and clinical outcome. Journal of the Medical Association of Thailand = Chotmaihet thangphaet. 2008 Jun
[PubMed PMID: 18697384]
Level 2 (mid-level) evidence
[12]
Chaudhry IA,Elkhamry SM,Al-Rashed W,Bosley TM, Carotid cavernous fistula: ophthalmological implications. Middle East African journal of ophthalmology. 2009 Apr
[PubMed PMID: 20142962]