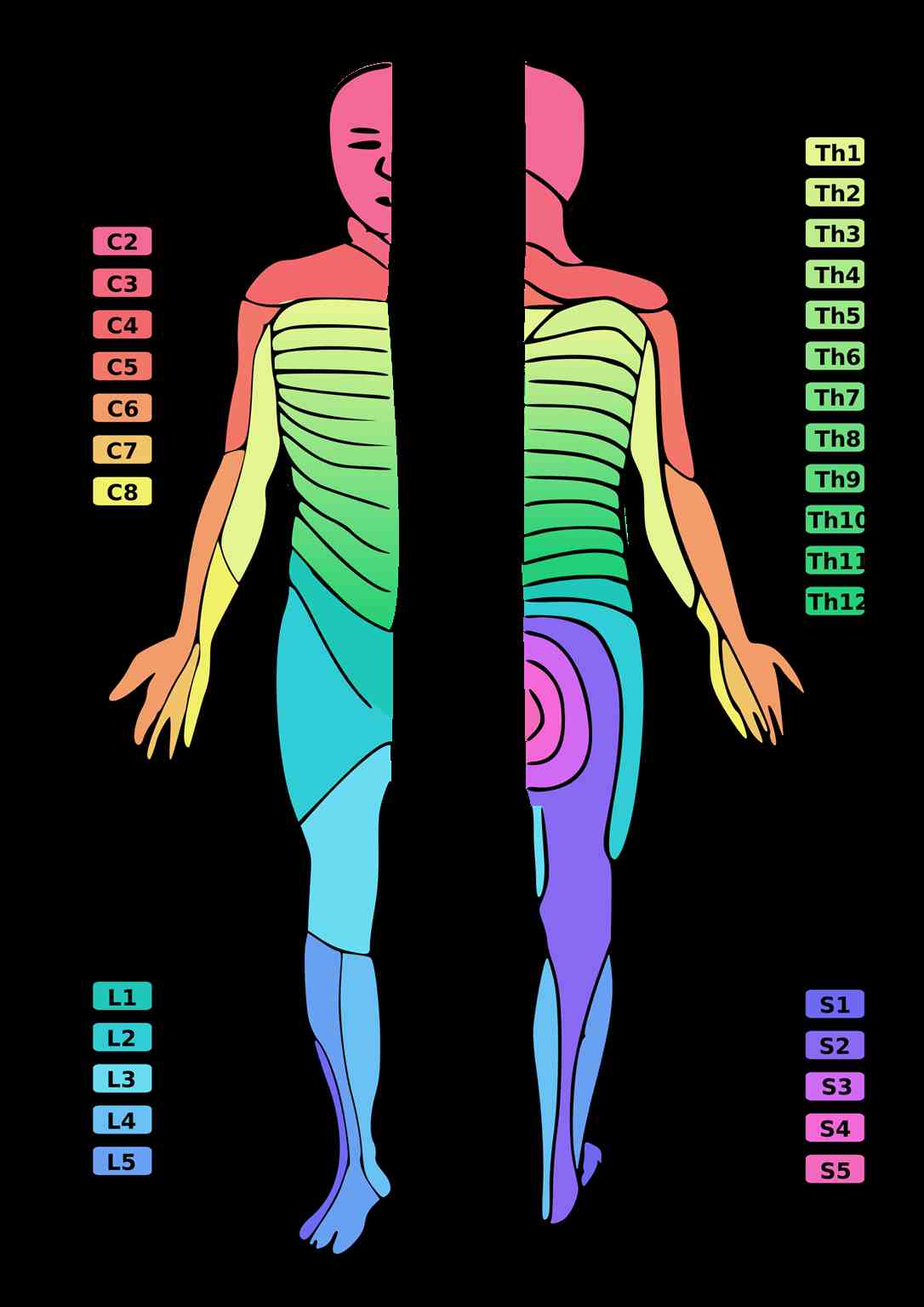
[1]
Watson JC, Dyck PJ. Peripheral Neuropathy: A Practical Approach to Diagnosis and Symptom Management. Mayo Clinic proceedings. 2015 Jul:90(7):940-51. doi: 10.1016/j.mayocp.2015.05.004. Epub
[PubMed PMID: 26141332]
[3]
Schraut NB, Walton S, Bou Monsef J, Shott S, Serici A, Soulii L, Amirouche F, Gonzalez MH, Kerns JM. What Protects Certain Nerves from Stretch Injury? Anatomical record (Hoboken, N.J. : 2007). 2016 Jan:299(1):111-7. doi: 10.1002/ar.23286. Epub 2015 Nov 25
[PubMed PMID: 26529568]
[4]
Gebhart GF, Bielefeldt K. Physiology of Visceral Pain. Comprehensive Physiology. 2016 Sep 15:6(4):1609-1633. doi: 10.1002/cphy.c150049. Epub 2016 Sep 15
[PubMed PMID: 27783853]
[5]
Spencer NJ, Zagorodnyuk V, Brookes SJ, Hibberd T. Spinal afferent nerve endings in visceral organs: recent advances. American journal of physiology. Gastrointestinal and liver physiology. 2016 Dec 1:311(6):G1056-G1063. doi: 10.1152/ajpgi.00319.2016. Epub 2016 Nov 17
[PubMed PMID: 27856418]
Level 3 (low-level) evidence
[6]
Yuan Q, Sun L, Yu H, An C. Human microvascular endothelial cell promotes the development of dorsal root ganglion neurons via BDNF pathway in a co-culture system. Bioscience, biotechnology, and biochemistry. 2017 Jul:81(7):1335-1342. doi: 10.1080/09168451.2017.1313695. Epub 2017 Apr 10
[PubMed PMID: 28394221]
[7]
Chao YC, Xie F, Li X, Guo R, Yang N, Zhang C, Shi R, Guan Y, Yue Y, Wang Y. Demethylation regulation of BDNF gene expression in dorsal root ganglion neurons is implicated in opioid-induced pain hypersensitivity in rats. Neurochemistry international. 2016 Jul:97():91-8. doi: 10.1016/j.neuint.2016.03.007. Epub 2016 Mar 10
[PubMed PMID: 26970395]
[8]
Qureshi AI, Saleem MA, Ahrar A, Raja F. Imaging of the Vasa Nervorum Using Contrast-Enhanced Ultrasound. Journal of neuroimaging : official journal of the American Society of Neuroimaging. 2017 Nov:27(6):583-588. doi: 10.1111/jon.12429. Epub 2017 Feb 14
[PubMed PMID: 28195441]
[9]
Cellek S, Cameron NE, Cotter MA, Muneer A. Pathophysiology of diabetic erectile dysfunction: potential contribution of vasa nervorum and advanced glycation endproducts. International journal of impotence research. 2013 Jan:25(1):1-6. doi: 10.1038/ijir.2012.30. Epub 2012 Aug 23
[PubMed PMID: 22914567]
[10]
Ishibe K, Tamatsu Y, Miura M, Shimada K. Morphological study of the vasa nervorum in the peripheral branch of human facial nerve. Okajimas folia anatomica Japonica. 2011 Nov:88(3):111-9
[PubMed PMID: 22519070]
[11]
Jeon SK, Paik DJ, Hwang YI. Variations in sural nerve formation pattern and distribution on the dorsum of the foot. Clinical anatomy (New York, N.Y.). 2017 May:30(4):525-532. doi: 10.1002/ca.22873. Epub 2017 Apr 8
[PubMed PMID: 28281304]
[12]
Eid EM, Hegazy AM. Anatomical variations of the human sural nerve and its role in clinical and surgical procedures. Clinical anatomy (New York, N.Y.). 2011 Mar:24(2):237-45. doi: 10.1002/ca.21068. Epub 2010 Oct 14
[PubMed PMID: 20949489]
[13]
Davidovich ER, Nascimento OJ. Superficial radial nerve-lateral antebrachial cutaneous nerve anatomic variation. Brain and behavior. 2014 Jan:4(1):70-4. doi: 10.1002/brb3.195. Epub 2013 Dec 1
[PubMed PMID: 24653956]
[14]
Guru A, Kumar N, Ravindra Shanthakumar S, Patil J, Nayak Badagabettu S, Aithal Padur A, Nelluri VM. Anatomical Study of the Ulnar Nerve Variations at High Humeral Level and Their Possible Clinical and Diagnostic Implications. Anatomy research international. 2015:2015():378063. doi: 10.1155/2015/378063. Epub 2015 Jul 12
[PubMed PMID: 26246909]
[15]
Falconer D, Spinner M. Anatomic variations in the motor and sensory supply of the thumb. Clinical orthopaedics and related research. 1985 May:(195):83-96
[PubMed PMID: 3978968]
[16]
Bas H, Kleinert JM. Anatomic variations in sensory innervation of the hand and digits. The Journal of hand surgery. 1999 Nov:24(6):1171-84
[PubMed PMID: 10584938]
[17]
Raducha JE, Cohen B, Blood T, Katarincic J. A Review of Brachial Plexus Birth Palsy: Injury and Rehabilitation. Rhode Island medical journal (2013). 2017 Nov 1:100(11):17-21
[PubMed PMID: 29088569]
[18]
Kubiak CA, Kung TA, Brown DL, Cederna PS, Kemp SWP. State-of-the-Art Techniques in Treating Peripheral Nerve Injury. Plastic and reconstructive surgery. 2018 Mar:141(3):702-710. doi: 10.1097/PRS.0000000000004121. Epub
[PubMed PMID: 29140901]
[19]
Middleton SD, Anakwe RE. Carpal tunnel syndrome. BMJ (Clinical research ed.). 2014 Nov 6:349():g6437. doi: 10.1136/bmj.g6437. Epub 2014 Nov 6
[PubMed PMID: 25378457]
[20]
Levi AD, Ross AL, Cuartas E, Qadir R, Temple HT. The surgical management of symptomatic peripheral nerve sheath tumors. Neurosurgery. 2010 Apr:66(4):833-40. doi: 10.1227/01.NEU.0000367636.91555.70. Epub
[PubMed PMID: 20190660]
[21]
Stratton JA, Assinck P, Sinha S, Kumar R, Moulson A, Patrick N, Raharjo E, Chan JA, Midha R, Tetzlaff W, Biernaskie J. Factors Within the Endoneurial Microenvironment Act to Suppress Tumorigenesis of MPNST. Frontiers in cellular neuroscience. 2018:12():356. doi: 10.3389/fncel.2018.00356. Epub 2018 Oct 11
[PubMed PMID: 30364248]
[22]
Muir D. The potentiation of peripheral nerve sheaths in regeneration and repair. Experimental neurology. 2010 May:223(1):102-11. doi: 10.1016/j.expneurol.2009.05.038. Epub 2009 Jun 6
[PubMed PMID: 19505459]
[23]
Scholz T, Krichevsky A, Sumarto A, Jaffurs D, Wirth GA, Paydar K, Evans GR. Peripheral nerve injuries: an international survey of current treatments and future perspectives. Journal of reconstructive microsurgery. 2009 Jul:25(6):339-44. doi: 10.1055/s-0029-1215529. Epub 2009 Mar 19
[PubMed PMID: 19301234]
Level 3 (low-level) evidence
[24]
Raducha JE, Gil JA, DeFroda SF, Wawrzynski J, Weiss AC. An Evidence-Based Approach to the Differentiation of Compressive Neuropathy from Polysensory Neuropathy in the Upper Extremity. JBJS reviews. 2017 Oct:5(10):e9. doi: 10.2106/JBJS.RVW.17.00028. Epub
[PubMed PMID: 29087965]
[25]
Kaya Y, Sarikcioglu L. Sir Herbert Seddon (1903-1977) and his classification scheme for peripheral nerve injury. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2015 Feb:31(2):177-80. doi: 10.1007/s00381-014-2560-y. Epub 2014 Oct 1
[PubMed PMID: 25269543]
[26]
Torg JS. Cervical spinal stenosis with cord neurapraxia and transient quadriplegia. Sports medicine (Auckland, N.Z.). 1995 Dec:20(6):429-34
[PubMed PMID: 8614762]
[27]
Wang E, Inaba K, Byerly S, Escamilla D, Cho J, Carey J, Stevanovic M, Ghiassi A, Demetriades D. Optimal timing for repair of peripheral nerve injuries. The journal of trauma and acute care surgery. 2017 Nov:83(5):875-881. doi: 10.1097/TA.0000000000001570. Epub
[PubMed PMID: 28590354]
[28]
Schloss J, Colosimo M. B Vitamin Complex and Chemotherapy-Induced Peripheral Neuropathy. Current oncology reports. 2017 Oct 5:19(12):76. doi: 10.1007/s11912-017-0636-z. Epub 2017 Oct 5
[PubMed PMID: 28983799]
[29]
Ramchandren S. Charcot-Marie-Tooth Disease and Other Genetic Polyneuropathies. Continuum (Minneapolis, Minn.). 2017 Oct:23(5, Peripheral Nerve and Motor Neuron Disorders):1360-1377. doi: 10.1212/CON.0000000000000529. Epub
[PubMed PMID: 28968366]
[30]
Santos DFD, Mendonça MR, Antunes DE, Sabino EFP, Pereira RC, Goulart LR, Goulart IMB. Revisiting primary neural leprosy: Clinical, serological, molecular, and neurophysiological aspects. PLoS neglected tropical diseases. 2017 Nov:11(11):e0006086. doi: 10.1371/journal.pntd.0006086. Epub 2017 Nov 27
[PubMed PMID: 29176796]