Continuing Education Activity
Sleep is the natural periodic suspension of consciousness characterized by lessened consciousness and slowed metabolism. The sleep-wake cycle is one of the most important circadian rhythms, which alternates in a lawful periodic fashion lasting for about 24 hours. Sleep is characterized by relative immobility and reduced responsiveness to environmental stimuli. This in contrast to the state of wakefulness which is characterized by presumably purposeful motor activity and the ability to respond to environmental stimuli appropriately. This activity reviews the electroencephalographic (EEG) findings of normal sleep patterns as well as abnormal sleep and discusses the role of the interprofessional team in educating patients on when it may be necessary to seek professional intervention if abnormal sleep patterns develop.
Objectives:
- Explain natural sleep.
- Describe and compare sleep versus wakefulness.
- Review normal sleep patterns compared to abnormal sleep on an EEG.
- Outline EEG normal sleep patterns as well as abnormal sleep and address the role of the interprofessional team in educating patients on when it may be necessary to seek professional intervention if abnormal sleep patterns develop.
Introduction
Sleep is the natural periodic suspension of consciousness characterized by lessened consciousness and slowed metabolism. The sleep-wake cycle is one of the most important circadian rhythms, which alternates in a lawful periodic fashion lasting for about 24 hours.[1] Sleep is characterized by relative immobility and reduced responsiveness to environmental stimuli. This in contrast to the state of wakefulness which is characterized by presumably purposeful motor activity and the ability to respond to environmental stimuli appropriately. Nature provides limited tolerance to the disturbance in sleep-wake cycling, leading to disastrous consequences.[2] This wrath has never been better experienced in human history as much as it is now. With the turn of the 19th century, the invention of the light bulb by Edison, transmeridian travel, and shift work have significantly contributed to the development of a new group of sleep problems described as shift work sleep disorders.[3]
Function
Different states of vigilance like waking and sleep states with or without dreams have been identified since ancient times. The Upanishads (approximately 1000 BCE) have described the different states of consciousness. The Hippocratic school (400 BCE) had understood the role of sleep in promoting good health.[4] Lucretius, as early as the first century BCE, described the deafferentation theory of sleep [1988 translation of Lucretius’ book On the Nature of the Universe [55 BCE]); the same was revived in the early 19th century by Macnish (Macnish, 1830) and Purkinje.[5] Subsequent developments in this field were carried out by Bremer, Moruzzi, and Kleitman, whose works recognized that sleep is an active, life-supporting process contrary to the long-held belief that sleep is simply a passive process.[6]
There are many theories on the functions of sleep, even though specific research has not validated these. This includes the early theory of restorative function, which involves the growth and changes in metabolism. There are contradictory findings on the restorative effects of post-exercise sleep–molecular studies on the cell division and DNA transcription function relative to circadian rhythms on various tissues. However, researchers generally agree that there are beneficial effects on neuronal function and behavior.[7]
There are theories on energy conservation to reduce energy expenditure by lowering metabolism and thermoregulation through hypothalamic nuclei. There are cross pathways of circadian rhythms and energy metabolism [8]; however, during REM sleep, there is increased whole-body oxygen consumption.[9]
Researchers also think that sleep brings about memory consolidation and the plasticity of the cognitive neural networks. Learning, behavior, and cognitive performance are improved after sleep, as evidenced by functional magnetic resonance imaging on the visuomotor task performance after normal sleep.[10][11]
This short article mainly covers the EEG aspects of sleep to familiarize the reader with terminology and specific characteristics of various sleep stages.
Issues of Concern
Sleep disorders affect humans of all ages. Sleep disorders can sometimes be manifestations of dysfunction of other organ systems and manifestations of abnormalities in neuropsychiatric functioning. This projects into loops of cascading derangements as disordered sleep affect brain function. Understanding the pathophysiology of sleep disorders is crucial in the diagnosis and management of disorders like attention deficit hyperactivity disorder (ADHD), depression, obesity, Parkinson disease, and epilepsy, to name a few.
Clinical Significance
Sleep Staging
The humble beginnings in sleep medicine started with a mere observation and description of various events occurring during sleep and wake. However, it was not until the development of the field of electrophysiology and the invention of electroencephalography (EEG) recordings by Hans Berger in the early part of the 20th century that we began to understand better the complexity of the brain mechanisms characterizing sleep and wake states. Sleep goes through structured and organized cycles through various stages.
The initial overnight sleep recordings were performed by Loomes and his colleagues as they faced the challenging task of describing typical sleep patterns in normal individuals. Several groups improvised this and, in turn, gave rise to the beginnings of sleep staging.[12] However, it was only 17 years later that Aserinsky recognized rapid eye movement (REM) sleep. This led to the birth of modern methods of sleep staging.[13]
In 1968, a committee of experts chaired by Rechtschaffen and Kales established the rules for the scoring of sleep in normal human adults. From this coding, 5 sleep stages were identified: 1 REM stage and 4 NREM sleep stages. Each stage consists of a number of physiological variables, which tend to occur in concert. Subsequently, in 2004, a revision of the sleep scoring rules was commissioned by the American Academy of Sleep Medicine (AASM)[14], which included rules for the scoring of arousals, respiratory events, sleep-related movement disorders, and cardiac events.[15] The magnitude and distribution of the standard sleep parameters reflect the macrostructure of sleep.
Macrostructure of Sleep
Based on sleep macrostructure, sleep can be classified into 2 main stages: non-rapid eye movement (NREM) and rapid eye movement (REM) sleep. Typically, as one goes to sleep, the low-voltage fast EEG pattern of wakefulness gradually gives way to slower frequencies, as NREM sleep goes from stage N1 (decrease in alpha) to stage N2 (spindles, K-complexes) to stage N3 (increasing amplitude and regularity of delta rhythm). Stage N3 is referred to as slow-wave sleep (SWS). SWS is interrupted by periods of rapid eye movement (REM, i.e., active or paradoxical) sleep. Polysomnography (PSG) is a multiparametric study that has been traditionally used to assess the architecture of sleep.
Sleep goes through multiple discrete cycles of NREM and REM sleep through any given night. In normal adults, each cycle lasts for about 90 to 120 minutes, and there are about 4 to 5 such cycles that occur during a normal 8 hour night sleep. The percentage of NREM sleep is maximum in the first part of the night, while REM sleep predominates in the second half.
Stage wake (W) is characterized by the presence of a predominant beta rhythm over the anterior leads, and there is a posterior progression to a posterior dominant alpha rhythm over the occipital regions. This anteroposterior progression is best observed with the eyes closed and is attenuated by eye-opening. Eye blinks are frequently observed in this stage which appear as conjugate eye movements consisting of 0.5 to 2 Hz. During the transition to drowsiness, one of the first things to appear is slow lateral eye movements typically less than 0.5 Hz, and there is greater prominence of alpha rhythm with intermittent beta rhythm.
Stage 1 (N1) is characterized typically by the disappearance of the alpha rhythm and appearance of roving eye movements, which are slow, conjugate, to and fro deflections usually lasting approximately 500 milliseconds. The EEG shows medium amplitude, mixed frequency predominantly of 4 to 7 Hz activity, and irregularly spaced bursts of slow waves. There is an appearance of vertex sharp transients (VST) which are defined as sharply contoured, bilateral synchronous waves with maximum amplitude over the central derivations, although children may show parietal dominance. The amplitude may vary on either side, and they usually last for fewer than 0.5 seconds. They are usually isolated and appear at irregular intervals both spontaneously as well as on the application of alerting stimuli. We also see the appearance of positive occipital sharp transients of sleep (POSTS), which are either mono or biphasic, positive, triangular waves most prominent in the occipital head regions. Alerting during N1 can lead to a brief recurrence of the alpha rhythm. EMG shows reduced muscle activity.
Stage 2 (N2) is characterized by the presence of bilaterally synchronous theta activity accompanied by sleep spindles or K-complexes, or both. K complexes are defined by the occurrence of a complex pattern of negative sharp wave immediately followed by a positive wave (V-shaped) standing out from the background EEG, lasting 0.5 seconds, and is most prominent in the fronto-central derivations. For arousal to be associated with the K complex, it should commence no more than 1 second after the termination of the K complex. Sleep spindles are defined as distinct 12 to 14 Hz waves having frequencies of 11 to 16 Hz (most commonly 12 to 14 Hz) with a duration of greater than equal to 0.5 seconds, usually maximal in amplitude in the central derivations.
Stage 3 (N3) is characterized by high amplitude, delta slowing in the range of 0.5 to 2 Hz with amplitudes of equal to 75 microV as measured over the fronto-central derivations. K-complexes and sleep spindles may be present, but POSTs are rare. Typically, N3 sleep is scored if slowing is seen in 20% of the epoch. N3 sleep occurs most frequently during the first one-third of the night, and clinically this can be important as NREM parasomnias such as sleepwalking and night terrors are typically seen during this period. Stage REM (R) is characterized by the presence of rapid eye movements (REM), which are conjugate, irregular, and sharply contoured eye movements with an initial phase deflection usually lasting less than 500 ms. We also see diminished EMG tone and is usually the lowest of the entire recording. Sawtooth waves are seen, which are described as drains of sharply contoured or triangular, often serrated waves of 2 to 6 Hz with maximal amplitude over the central derivations and often, but not always preceded by a burst of rapid eye movements. The threshold for arousal by auditory stimuli tends to be the highest during REM. Typically, the R stage of sleep is present predominantly in the last one-third of the night and is the period where the REM parasomnias such as nightmares are typically seen. Stage R can be further subdivided into a phasic REM and a tonic REM stage. The phasic REM stage is a sympathetically driven sleep state characterized by the presence of rapid eye movements, intermittent muscle twitches, and variations in breathing patterns. The tonic REM, on the other hand, is a parasympathetically driven sleep state and is characterized by the absence of rapid eye movements.
Traditional visual-stage scoring of PSG records has provided valuable descriptions of sleep macro-architectural abnormalities in a variety of sleep disorders. However, they do not provide information about the EEG frequency characteristics or rhythmicity that underlie sleep disturbances. Further, the underlying assumption in stage scoring algorithms is that sleep is a discontinuous and discretely bounded process, an assumption that has not been supported by recent data.
Microstructural Analysis of Sleep
Transient EEG phenomena lasting less than the scoring epoch (phasic events) have been described within the sleep recordings allowing identification of what is known as the microstructure of sleep.[16] The two most commonly used methods to study the microstructure of sleep include cyclic alternating pattern (CAP) analysis and arousal paradigm.
Arousal Analysis
In 1992, the American Sleep Disorders Association (ASDA) proposed a definition of arousal[17] independent of the R and K staging. According to the ASDA criteria, EEG arousals appear as sudden frequency shifts towards faster rhythms (theta, alpha, beta, but not sigma) that briefly replace the sleep stage background. In normal subjects, the mean duration of arousals remains unmodified across the lifespan (average length of about 15 seconds throughout TST), but the increase in number with age[18] is considered as the physiological basis of sleep fragility in the elderly.[19] In conditions of disturbed sleep, arousals have been investigated, especially in sleep-related breathing disorders and in insomniac patients. There is, however, consolidated literature according to which arousals and other related phenomena represent spontaneous manifestations of physiological sleep.[20][21][22]
CAP Analysis
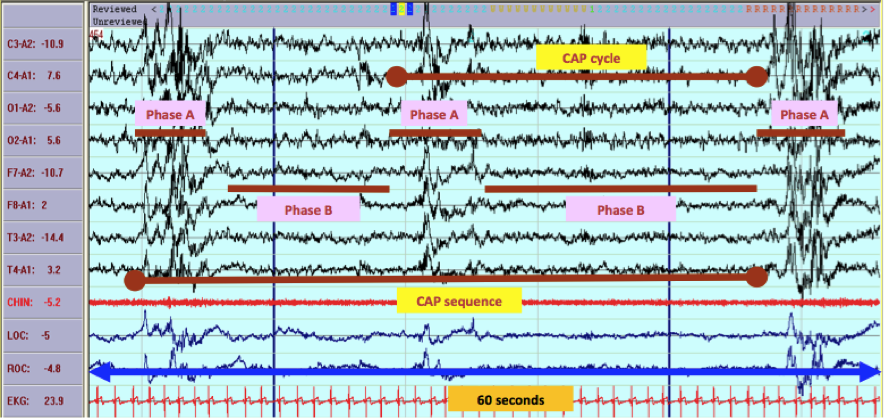
CAP is an EEG phenomenon organized in sequences that occupy wide sections within NREM sleep.[23] During CAP, the EEG rhythms of sleep oscillate with periodic excitatory (phase A) and inhibitory (phase B) swings. CAP is a major marker of arousal instability that accompanies the sleep-wake transitional phases[24], and researchers think it is a substrate for the emergence of various sleep activated neurological disorders. Repetitive clusters of stereotyped EEG features separated by time-equivalent intervals of background activity and include at least 2 consecutive CAP cycles identify a CAP sequence. The CAP cycle consists of a phase A (composed of transient EEG elements) and a phase B (interval of theta/delta activity that separates 2 successive A phases, with an interval equal to 1 minute) (Figure 1). Each phase of CAP may last for 2 to 60 seconds. All CAP sequences begin with phase A and ended with phase B.[25] Based on the reciprocal proportion of high-voltage slow waves (EEG synchrony) and low-amplitude fast rhythms (EEG desynchrony) throughout the entire phase A duration, 3 subtypes of A phases corresponding to different levels of neurophysiologic activation are distinguished: subtype A1 (predominance of EEG synchrony), subtype A2 (balanced mixture of EEG synchrony and desynchrony), and subtype A3 (predominance of EEG desynchrony). When the interval between 2 consecutive A phases exceeds 60 seconds, the CAP sequence ends, and sleep entered into the non-CAP (NCAP) mode is characterized by stable, ongoing EEG rhythms with very few and randomly distributed arousal-related phasic events.[26]
Other Issues
Disorders of Sleep
It is now commonly accepted that sleep deprivation is a major cause of accidents, including traffic accidents.[27][28][29] There are around 90 distinct sleep disorders characterized by a disturbance in the patient's amount of sleep, quality and timing of sleep, behaviors, or physiological conditions associated with sleep.
The effect of drugs on sleep and sleep cycles has been another concern in the treatment of many disorders, including Parkinson, dementia, epilepsy, anxiety, and depression. There is quality of life and safety concerns in the management of sleep disorders.
Hypnagogic Hypersynchrony
In children, during drowsiness and early sleep, EEG may show very high amplitude slow waves or theta activity with occasional sharply contoured waveforms. This can mimic the 3 Hz spike-wave discharges seen in primary generalized epilepsies. It is important to be aware of the unique EEG characteristics of sleep in childhood to reduce the misleading diagnosis of epilepsy[30]
Enhancing Healthcare Team Outcomes
As mentioned, normal sleep plays an important function in human life. The changes in occupational technology, workplace ergonomics, and safety and risk reduction strategies are important for ensuring the prevention of injuries and negative effects of sleep deprivation and disturbance in circadian function. Adequate strategies to assess sleep disorders and employee wellness is an occupational safety issue that requires administrative and regulatory mandates. For children, the participation of teachers and school health staff ensures that sleep disorders and attention and learning issues are adequately evaluated. Furthermore, if sleep disorders are present, early referrals to sleep medicine specialists, psychiatrists, and/or neurologists should be made.
Important EEG features of various stages of sleep help sleep technologists to score the stages and to help the sleep specialists to assess the sleep disorders accurately. Sleep disorder management is interprofessional. It involves ear, nose, and throat specialists, pulmonologists, sleep medicine specialists, and psychiatrists working together to help patients and families to improve behavior, cognitive function, and overall health.[31][32][33]