Continuing Education Activity
Asbestosis is an interstitial lung disease caused by the inhalation of asbestos fibers. Because of their high electrical and thermal resistance and low cost, asbestos fibers have been historically chosen for commercial use in construction, shipping, mining, and aerospace engineering. This activity describes the evaluation and management of patients with asbestosis and highlights the role of the interprofessional team in improving care for patients with this condition.
Objectives:
Identify the etiology of asbestosis.
Determine the appropriate evaluation of asbestosis.
Identify the management options available for asbestosis.
Communicate the interprofessional team strategies for improving care coordination and communication to advance asbestosis and improve outcomes.
Introduction
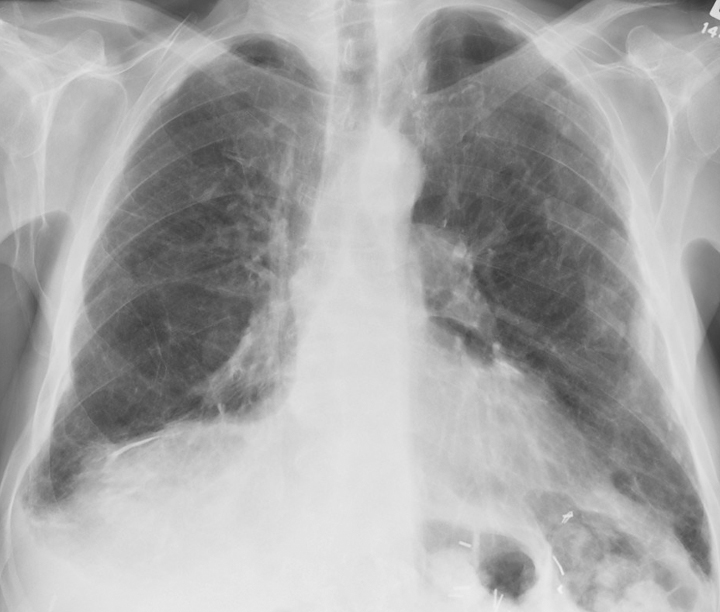
Asbestosis is an interstitial lung disease caused by inhaling asbestos fibers (See Image. X-ray, Lungs, Asbestos, Anterior View). These fibers are mineral silicates (mainly hydrated magnesium silicates) and are classified into 2 main categories based on their shape: serpentine and amphibole.[1] Serpentine fibers include curly and flexible chrysolites and are less pathogenic than amphibole fibers. Chrysolite, being more flexible, curvy, and soluble, settles in the upper part of the respiratory tract. The mucociliary function is more prominent in the upper respiratory tract, so chrysolite fibers are easily removed. Amphiboles (crocidolite, amosite, tremolite, and anthophyllite) are straight, stiff, more brittle fibers. They are more toxic than serpentine fibers, which are less soluble and straight. They usually align with the airstream and penetrate the epithelium deeper into the lungs and interstitium. Asbestos fibers have been historically chosen for construction, shipping, mining, and aerospace engineering commercial use because of their high electrical and thermal resistance and low cost.
Etiology
There are 3 main forms of asbestos exposure. Direct work-related environmental exposure is common among workers at shipyard, mining, aerospace, etc. Bystander exposure is the second form commonly seen in professionals like electricians, masons, and painters. The third and most common form of asbestos exposure is general community exposure, such as using asbestos for road surfaces, playground material, landfills, and chemical paints. The disease is dose-dependent; exposure is higher in the first group and lower in the second and third forms of exposure. Also, the amphibole variety's disease risk is higher than the serpentine asbestosis fibers.
Epidemiology
The prevalence of the disease is higher in those disciplines where the intensity of exposure is high. A study conducted in Okayama, Japan, among construction workers, revealed that workers working in the installation of the asbestos board have the highest number of cases (39.1%), followed by sprayer of asbestosis (38.5%). Also, the longer the duration of exposure, the more the chances of asbestosis.[2] In the UK, insulation workers have the highest risk of asbestosis, followed by asbestos stripping.[3] It is estimated that asbestos causes around 55000 deaths per year in the world.[4] More prevalent in construction workers.[3]
The latent period for benign disease is shorter than other pulmonary complications. The incidence of pleural effusion is associated with the site of exposure and was found to be 9 cases per 1000 in heavily exposed groups. Although mesothelioma is less prevalent than lung cancer, over 2000 cases per year in the twenty-first century were reported in the United States of America. The incidence of lung cancer is comparatively higher than that of mesothelioma. The use of asbestos in developed countries has been reduced, but use in developing countries like Nepal and Pakistan is considerably high.
Pathophysiology
Interstitial fibrosis is regarded as the principal pathogenic mechanism of asbestosis. It is believed that after the deposition and transmigration of asbestos fibers in the lung, macrophages accumulate followed by fibroblasts that lay the foundation for fibrosis. Reactive oxygen species produced by immune cells and phagocytes in response to asbestos fibers result in oxidative injury. These reactive oxygen species and transepithelial migration of fibers injure the type 1 alveolar cells. Injured epithelial cells also produce fibroblast growth factor-beta, which induces fibrosis. In an attempt to phagocytose the foreign body, macrophages produce inflammatory mediators such as tissue necrosis factors, interleukins, and stimulation of the phospholipase C pathway.[5][6] These mediators are key in stimulating the other cells, like lymphocytes and myofibroblasts. This leads to the proliferation of fibroblasts and an increase in the number of cells in the matrix by about 2-fold. Macrophages also produce fibroblast growth factors, platelet-derived growth factors, and insulin-like growth factors that cause fibrosis. Plasminogen activator produced by macrophages degrades the matrix glycoproteins, further damaging the interstitium.
As this is a progressive disease, fibrosis increases over time. Asbestos fibers also activate serum complement C5a, a chemotactic factor for macrophages.[7] In most cases, asbestos fibers coated by other toxins, including transition metals, induce the formation of reactive oxygen species. Iron-coated on these fibers produces hydroxyl ions in 1 cell-free system, causing free radicle injury.[6]
A recent investigation revealed that the length of fibers directly relates to pathogenesis. Longer fibers are more potent than shorter fibers in stimulating the NF-kB pathway and gene promoter activity.[5] Exposure to amphibole is linked to autoantibody production. Patients with positive antinuclear antibody tests are believed to have more chances of developing interstitial and pleural abnormalities.[8] In addition to cellular and fibrotic reactions, asbestos possibly functions as a tumor initiator and promoter. Amphibole type has more tendency for carcinogenesis of pleura. The severity of asbestos-related pulmonary fibrosis is related to the total dose of exposure.
Histopathology
On gross examination, the visceral pleura is markedly thickened, especially on the lateral and diaphragmatic surface of the lung, with localized fibrous plaques and pleural effusions. Diffuse fibrosis of the lower lobe of the lung is visible on the cut section.
Asbestosis is marked by interstitial fibrosis with characteristic asbestos and ferruginous bodies on microscopic examination. The presence of both asbestos bodies and ferruginous bodies helps pathologists differentiate between asbestosis and interstitial lung fibrosis. Asbestos bodies are golden yellow, beaded rod-like structures with a translucent center. It is formed when asbestos fibers get coated with iron-containing proteinaceous material. A ferruginous body indicates that the patient may have had prior asbestos exposure and appears as golden-brown fusiform rods resembling dumbells. Fibrosis distorts the lung parenchyma architecture, which causes the formation of enlarged air space surrounded by a thick fibrous wall and hyperplastic type 2 pneumocytes called a honeycomb appearance.
History and Physical
The history of occupation is crucial for both diagnosis and management. A history of asbestosis exposure helps exclude other chronic interstitial lung diseases. Usually, there is a history of 10 to 20 years of asbestos exposure and progressively worsening dyspnea. Cough with sputum and wheezing are unusual, though, if present, they are mainly associated with smoking. The patient may complain of chest discomfort due to heart failure following pulmonary hypertension. The severity of the disease depends on the duration and intensity of exposure and whether the patient has direct occupational contact with asbestos. History of smoking and dyspnea on exertion are important, and nonproductive cough is quite common. History of loss of appetite and weight, hemoptysis indicates the suspicion of lung tumors. Gradual onset of localized pain or breathlessness radiating to the shoulder may be evident in pleural involvement.[9]
Physical examination reveals clubbing in 32% to 42%, asbestos warts, and reduced chest expansion due to restrictive lung disease (38%). Bibasilar rales are best auscultated at lower lateral and basal areas.[9][10] In advanced disease, the patient may show signs of cor pulmonale, such as pedal edema, jugular venous distension, right ventricular heave, hepatojugular reflux, and cyanosis. These signs are mainly due to right-sided heart failure from pulmonary vascular remodeling.
Evaluation
The diagnosis of asbestosis is largely clinical. First, the history of asbestos exposure is central to the diagnosis. The overall clinical picture is characterized by progressive restrictive pulmonary disease with interstitial fibrosis on radiography. The pulmonary function test reveals characteristic restrictive disease.[3]
Pulmonary Function Tests
- Spirometry and Lung Volum: This includes forced vital capacity (FVC), forced expiratory volume in 1 sec (FEV1), total lung capacity (TLC), functional residual capacity, and residual volume. Like any other restrictive lung disease, all earlier-mentioned lung volumes are reduced. FEV1/FVC ratio is either normal or increased.
- Diffusing Capacity: Total carbon monoxide diffusion capacity (DLCO) reduction is a common but nonspecific finding. Reduced total carbon monoxide diffusion capacity (DLCO) is manifested earlier.[11] This is due to the mismatching of ventilation and perfusion (V/Q).
Arterial Blood Gas
Arterial oxygen tension (pao2) may be normal or reveal hypoxia and respiratory alkalosis.[12] Carbon dioxide retention is rare; if present indicates the end-stage disease, there may also be airflow obstruction due to small airway dysfunction. Nevertheless, it is important to note that asbestosis is a diagnosis of exclusion. It is clinically relevant to differentiate asbestosis from idiopathic pulmonary fibrosis (IPF) because both essentially have many similar presenting symptoms. Both asbestosis and IPF are characterized by progressive interstitial pulmonary fibrosis, with a restrictive lung disease picture in PFT. There are subtle differences between the 2 radiographically and histologically. It is important to differentiate between the 2 because distinctly targeted therapies are available, although ineffective.
Chest Radiograph and HRCT Chest
Chest radiograph shows diffuse reticulonodular infiltrates at the lung bases, causing shaggy heart borders. High-resolution computed tomography (HRCT) is often diagnostic of asbestosis.[13] HRCT shows ground-glass opacities, along with diffuse interstitial fibrosis in asbestosis, whereas, in idiopathic pulmonary fibrosis, there is evidence of patches of opacities. Pleural thickening and calcified pleural plaques in tomography are the hallmarks of the disease. Asbestosis mimics idiopathic pulmonary fibrosis radiographically, especially in CT. The important distinguishing point is that asbestosis begins more centrally and dissipates peripherally following a centrifugal pattern. In contrast, idiopathic pulmonary fibrosis begins peripherally, especially at the bases, and progresses centrally and upwards as the disease progresses. At least theoretically, IPF follows a centripetal pattern. Differentiating asbestosis and idiopathic pulmonary fibrosis (IPF) on CT can be challenging, and histopathology can be conclusive.
Lung Biopsy
It shows interstitial fibrosis with characteristically peribronchial fibrosis. The distribution of fibrosis in asbestosis is from the center to the periphery, ie, centrifugally. The microscopic view of asbestosis shows distinct asbestos bodies coated with iron-containing proteinaceous material and diffuse pulmonary interstitial fibrosis. Still, in contrast, idiopathic pulmonary fibrosis (IPF) displays patchy interstitial fibrosis. The profuse proliferation of fibroblasts, ie, fibroblastic foci and subsistence of early and late proliferative lesions (temporal heterogeneity), is well defined in IPF and rare or absent in asbestosis.
Bronchoscopy/VAT
Endobronchial, transbronchial, and video-assisted thoracoscopic (VAT) approaches can achieve a lung biopsy. The endobronchial biopsy is done with ultrasound-guided bronchoscopy to visualize the airway and adjacent structures. This technique has been routinely used in many centers due to its high diagnostic value and low risk. Endoscopically-guided forceps do transbronchial lung biopsy. Conventional transbronchial biopsy has a higher risk of complications and bleeding. The modern technique is the minimally invasive video-assisted thoracoscopic (VAT) guided biopsy. VAT has fewer complications, is more sensitive and specific, and has more diagnostic yield than transbronchial biopsy. So, VAT is preferred over transbronchial lung biopsy, though VAT has some discrepancies like cost and expertise requirement.[14]
Bronchoalveolar lavage has a limited role. Samples can be taken from suspected patients for cellular analysis of asbestos body count, inflammatory cells like macrophages, neutrophils, eosinophils, and dust particles. Asbestos body formation is more common in amphibole fibers, while chrysotile fibers have a shorter half-life and have fewer chances of asbestos body formation. So the absence of asbestos bodies in chrysotile asbestosis leads to a false-negative result.[15]
Biochemistry
Lab analysis shows elevated C-reactive protein, erythrocyte sedimentation rate, rheumatoid factor, and antinuclear antibodies.
Treatment / Management
Asbestosis has no specific treatment, so supportive care is the only option. Therefore, prevention is the best management. Monitoring the occupational environment and minimizing asbestos exposure are significant in asbestosis control.
Drug Therapy
Corticosteroid therapy aims to suppress the acute and chronic inflammatory process, thereby reducing lung damage, but the success rate is low. Steroids suppress the release of reactive species and mediators in the ongoing inflammatory process. Because of the lack of placebo-controlled trials, there is no direct evidence of the benefits of steroids in improving the survival of life. The proper dose and length of therapy of steroids are not known.[16] High-dose corticosteroids and other immunosuppressive drugs, such as azathioprine, have some roles in reducing the exacerbation of acute asbestosis. The starting dose of prednisolone is usually considered 0.5 to 1 mg per kg in a once-daily oral dose for 4 to 12 weeks. The patient is monitored and reevaluated. If the patient is stable and improving, the dose is tapered to 0.25 to 0.5 mg/kg for 4 to 12 weeks. However, some studies show that using agents like cyclophosphamide accelerates the fibrotic process, limiting its use. If the condition is not improving and the patient is unstable, then either other drugs are added, or steroid is withdrawn. Colchicine is found to be a mild antifibrotic agent.[17] Antibiotic therapy should be prompt for superimposed respiratory infections and immunization against pneumococcal pneumonia and influenza.
Patients with asbestosis sometimes has also a history of smoking, and they develop chronic obstructive pulmonary disease (COPD). In such cases, inhalers containing long-acting beta 2 agonists, inhaled corticosteroids, or long-acting muscarinic antagonists are also prescribed. Smoking cessation is also advised.
Oxygenation
Patients with hypoxemia (Pao2 less than 55 mmHg) at rest or on exertion should be supplemented with oxygen. Pulmonary rehabilitation and management of cor pulmonale have been shown to improve the quality of life as the disease progresses.
Surgery
Decortication of subpleural fibrosis by thoracotomy improves atelectasis. Pleurectomy can be performed in pleural fibrosis.[18] But if the collection of pleural fluid is rapid, then only palliative removal of fluids to relieve breathlessness should be done.[9]
Lung Transplantation
This is the ultimate treatment in severe asbestosis, where all other therapies have failed in the case of chronic and irreversible fibrosis.
Differential Diagnosis
Differentials of asbestosis include diseases involving the interstitium of the lungs, such as idiopathic pulmonary fibrosis, interstitial pneumonitis, rheumatoid arthritis, hypersensitive pneumonitis, pleuropulmonary fibroelastosis, drug-induced pneumonitis, combined pulmonary fibrosis, and emphysema. Diagnosis of asbestosis requires the exclusion of these differentials.
Non-specific Interstitial Pneumonitis
This may be idiopathic or associated with other connective tissue disorders. Lower lobe involvement, ground-glass opacities, and impairment of pulmonary functions mimic this condition with asbestosis, but female predominance and rare honeycombing help in differentiation. History of exposure and histopathology is important to rule out other similar conditions.[19]
Chronic Hypersensitivity Pneumonitis
It is an allergic reaction based on the antigen-antibody interaction following exposure to allergens. Clinical presentation of hypersensitive pneumonitis, such as dyspnea and cough, resembles asbestosis. So lung biopsy with histopathology evaluation is required. It involves interstitial inflammation with granulomas and giant cells, usually throughout the whole lung, compared to more pronounced fibrosis in the lower lobes of the lung in asbestosis.[20]
Rheumatic Disease
The rheumatic disease involves the interstitium of the lung in about 10% of cases. Unlike asbestosis, other systemic manifestations like arthritis, Raynaud phenomenon, muscle weakness, and skin changes are present in rheumatic disease.[21]
Drug-induced Interstitial Pneumonitis
Some drugs used for purposes other than pulmonary diseases have pulmonary toxicity involving interstitium as their side effects. Anagrelide, a phospholipase A2 inhibitor, lamotrigine, cyclophosphamide, bleomycin, nitrofurantoin, etc, are the drugs that cause pulmonary toxicity.[22][23] This interstitial lung toxicity may be confused with asbestosis, but the history of drug intake helps to distinguish the real cause of the disease.[24][25]
Pleuropulmonary Fibroelastosis
Pleuropulmonary fibroelastosis is a rare benign condition. Though this disease simulates asbestosis, this condition involves pleura and subpleural parenchyma almost simultaneously. Fibrosis is due to elastic fiber proliferation. However, in asbestosis, pleural involvement is not parallel to parenchymal fibrosis, as pleural involvement is usually the late presentation. Also, pleuropulmonary fibroelastosis may involve the upper lobe of the lungs.[26]
Silicosis
Silicosis has more tendency to undergo fibrosis than asbestosis, forming a whorled pattern of collagen fibers that can be appreciated in histology. Occupational history makes an easy way out to exclude silicosis. Fibrosis may also involve hilar lymph nodes and sometimes egg-shell calcification.[27]
Sarcoidosis
Sarcoidosis is a chronic granulomatous disease of the lung that involves the multisystem. CD4+ to CD8+ cells ratio is increased as in asbestosis, but it has a more pronounced increment than in asbestosis. Histopathology reveals noncaseating granuloma, asteroid bodies, and Schaumann bodies are characteristic of sarcoidosis not found in asbestosis.[28]
Coal Worker Pneumoconiosis (CWP)
It is also called black lung disease. It is the coal dust-induced progressive massive fibrosis disease. Cut section and biopsy of the lungs of workers working in coal mines show black patches mixed with fibrosis.[29]
Combined Pulmonary Fibrosis and Emphysema
Usually, emphysema is highly associated with smoking and occurs without fibrosis. The co-existence of fibrosis and emphysema was first established in 1990.[30] Upper lobe emphysema and lower lobe fibrosis are seen in some male smokers.
Prognosis
The severity of the disease depends upon the duration and intensity of exposure. More intense contact for a long period has a poor prognosis. Early intervention after the first symptom has a good outcome and prolongs survival. The condition worsens in acute exacerbations and superimposed infections. Radiologic screening in workers helps in picking the earliest abnormal changes.[31] Steroids have only symptomatic treatment. So, outcomes after steroid therapy do not always favor the alleviation of disease. Progression of the disease process can be stopped by removing the offenders. In later stages, the disease process becomes irreversible and may finally end with lung cancer. Patients with pleural involvement rarely survive more than a year after diagnosis has been made. But survival from the first symptom or earliest screening is a maximum of 4 years.[32] Radical surgery or radiotherapy of mesothelioma may aid in the extension of tumors to the chest wall.
Complications
Complications increase with the duration and intensity of exposure. Following complications occur in patients having asbestos exposure:
Respiratory Failure
Asbestosis is a restrictive lung disease characterized by the restricted filling of the lung. Total lung capacity and forced vital capacity reduce significantly. Patients complain of progressive dyspnea on exertion and cough. Deterioration of diffusing capacity and oxygenation is common. Pleural fibrosis prevents the expansion of the lung. Carbon dioxide retention is another hazard that leads to respiratory acidosis.[18] In many cases, benign pleural effusion occurs early, followed by pleural plaque formation. Effusion is generally bilateral and exudative and mostly remains asymptomatic. Fibrosis causes the derangement of the pulmonary vascular system, especially capillaries, which causes pulmonary hypertension. The decrease in diffusing capacity has a direct impact on hypoxemia.
Malignancy
Patients with asbestosis have a high risk of developing pleural malignancy. Patients who have low exposure to asbestos fibers have sequelae of asbestosis followed by malignancy. Still, those with intermediate or high-intensity exposure have a high chance of lung cancer, even in the absence of asbestosis. Lung cancer has more propensity to develop when the smoker is exposed to asbestos due to the additive effect of asbestos and cigarette smoke. Studies show that adenocarcinoma (primary bronchogenic carcinoma) is the major type of cancer that accounts for about 45%, followed by squamous cell carcinoma in about 42% of cases, and 13% is undifferentiated. Unlike fibrosis, most tumors are present in the upper lobes (69%) and only 13% in the lower lobes.[33] Mesothelioma of the pleura and peritoneum is common but is less prevalent than lung cancer. In contrast to bronchogenic carcinoma, the risk of mesothelioma does not increase with smoking in patients with asbestosis. Epidemiologic studies have explored that greater than 80% of mesothelioma is due to asbestos exposure.[34]
Heart and Other Organs
It was found that heart weight is increased due to hypertrophy of the right ventricle pumping against high pulmonary vascular resistance. More commonly, left auricle hypertrophy has been appreciated. Pulmonary and tricuspid valves are generally normal, but the mitral valve is fibrosed and fused with thickening chordae tendineae. Advanced asbestosis causes right heart failure, leading to cor pulmonale. The liver is congested in the centrilobular pattern due to the right-sided blood damming.[35]
Cancers of Other Organs
Cohort's study has unveiled that asbestosis is related to cancers of the gastrointestinal tract, ovaries, adrenals, larynx, and kidney. Gastrointestinal tract cancer is mainly due to exposure to asbestos through asbestos-lined cement water pipes.[36]
Consultations
Management of asbestosis requires a multidisciplinary team consisting of:
- Pulmonologist
- Radiologist
- Oncologist
- Thoracic surgeon
- Histopathologist
- Pharmacist
- Nurses
Deterrence and Patient Education
Asbestosis is an occupational disease, so it is more prevalent in workers. Prevention is the utmost step in the management of asbestosis. Despite the usefulness of asbestos, it is hazardous for long-term use and exposure. In the modern world, concern over the harmful effects is rising continuously. Research has been conducted to evaluate the effects of asbestos on health. Because it takes a long time to develop the disease as it is a progressive process, cessation of exposure stops the disease process. Avoiding potential risks, health education and safety training, monitoring of the work environment, periodic examination, and helping treatment to the workers to control the disease. Workers should be alarmed and have the right to know about the symptoms, outcomes of treatment, the toxicity of drugs, and health problems of asbestosis, and they should consult the health team as soon as possible. Patients suffering from asbestosis should stop smoking and minimize their exposure by changing their workplace or occupation.
Enhancing Healthcare Team Outcomes
Competent healthcare systems, in collaboration with other organizations and government bodies, should work together to control occupational diseases. The healthcare team has more diagnostic, therapeutic, and supportive care. So prevention or minimization of exposure necessitates government legislation to act accordingly, international labor organizations, the World Health Organization, and dust management teams. Intersectoral coordination greatly impacts optimizing the working environment and working hours. Applying engineering techniques to maintain appropriate working sites for optimum workers' health is essential. A healthcare team consisting of general providers, radiologists, pulmonologists, pharmacists, surgeons, oncologists, nurses, and histopathologists assists in making a proper diagnosis and appropriate treatment.[19] Proper diagnosis with the help of a multi-disciplinary healthcare team helps to devise the right way to the suitable treatment. Nursing care during the treatment has its importance. Complications of asbestosis require multimodality treatment options such as oncologists to treat lung cancer, surgeons for decortication and pleurectomy, etc.