Continuing Education Activity
Waterhouse Friderichsen syndrome is a rare but life-threatening disorder associated with bilateral adrenal hemorrhage. In many cases, it is caused by fulminant meningococcemia, but there are numerous etiologies. This activity describes the evaluation and management of Waterhouse Friderichsen syndrome and highlights the role of the healthcare team in managing patients with this condition.
Objectives:
- Identify the etiology of Waterhouse Friderichsen syndrome.
- Describe the pathophysiology of Waterhouse Friderichsen syndrome.
- Summarize the management of Waterhouse Friderichsen syndrome.
- Explain the importance of improving care coordination amongst the interprofessional team to improve outcomes for patients diagnosed with Waterhouse Friderichsen syndrome.
Introduction
Waterhouse-Friderichsen syndrome (WFS) is a rare clinical conundrum first described by Rupert Waterhouse and Carl Friderichsen as bilateral adrenal hemorrhage in the setting of bacterial sepsis among children in the first decade of the 20th century. Over the years, there have been reports of bilateral adrenal hemorrhage (AH) in association with multiple etiologies, including various systemic bacterial and viral infections. WFS has thus been used as a broader clinical term to address the adrenal insufficiency associated with bilateral adrenal hemorrhage. Although the adrenal insufficiency is mainly a feature in those with bilateral adrenal hemorrhage, there have been instances where the insufficiency occurred in unilateral hemorrhage. In these situations, the opposite adrenal gland was exhausted, i.e., the cortical lipoid was reduced or absent.[1]
Etiology
Waterhouse-Friderichsen syndrome was first described in cases of Neisseria meningitidis sepsis. Over the years, several bacterial and viral causes have correlated, which include and are not limited to, Streptococcus pneumonia,[2] Hemophilus influenzae,[2] Escherichia coli,[3] Staphylococcus aureus,[4] Group A beta-hemolytic Streptococcus,[5] Capnocytophaga canimorsus,[6][7] Enterobacter cloacae,[8] Pasteurella multocida,[9] Plesiomonas shigelloides,[10] Neisseria gonorrhoeae,[11] and Moraxella duplex.[12] Bilateral adrenal hemorrhage has also been reported with Rickettsia rickettsii,[13] Bacillus anthracis,[13] Treponema pallidum,[13] Legionella pneumophila,[13] and viral infections including Cytomegalovirus, Parvovirus B19, Epstein-Barr virus, and Varicella zoster virus.[4][14][15][16] In a study by Margaretten W, et al. performed in 51 children who died due to sepsis and bilateral adrenal hemorrhage, Pseudomonas aeruginosa was the most common pathogen identified.[14] While another study by Guarner J, et al. showed N. meningitidis as the most common bacteria associated with adrenal hemorrhage.[13] Other risk factors associated with WFS include the use of anticoagulants, thrombocytopenia, hypercoagulable states as heparin-induced thrombocytopenia[17], and antiphospholipid syndrome[3][18], trauma to the adrenals, postoperative state.
In a 25-year-study done at Mayo Clinic, trauma was the reported cause of adrenal hemorrhage in 4 out of 141 cases (2.8%). The hemorrhage was bilateral in 2 cases.[19] While in another study done over a 10-year-period, trauma was the etiology of adrenal hemorrhage in 28.7 % of cases. All of the patients but 1 had unilateral AH.[20]
3 (2.1%) patients experienced AH associated with anticoagulant therapy in the 25-year study.[19] AH as a complication of chronic anticoagulation in patients receiving warfarin alone without antiplatelet therapy was uncommon (5.4%, n=6). International normalized ratio (INR) among these patients were variable (mean = 3.13; SD=0.43; range=2.8-4.0).[20]
In the same 25-year-study, hemorrhage associated with heparin associated thrombocytopenia (HAT) and antiphospholipid antibody syndrome was present in 20 patients (14.2%). Twelve patients were shown to have a circulating lupus anticoagulant. Ten patients had heparin associated thrombocytopenia, and 2 of them had lupus anticoagulant as well.[19] Heparin-induced thrombocytopenia (HIT) comprised 9.6 % (n=11) of the total cohort in the other study; 10 of them (91%) developed HIT-related AH in the postoperative setting. All but one patient with HIT had bilateral AH, and all these ten patients exhibited acute adrenal insufficiency clinically.[20]
Epidemiology
Waterhouse Friderichsen syndrome is a rare condition seen in about 1 percent of routine autopsies.[21] No such prevalence studies have taken place so far. It is more common in children compared to adults.
Pathophysiology
Although the pathophysiology is unclear, various theories have been postulated to describe the adrenal hemorrhage seen in WFS. From an anatomical standpoint, the adrenal gland is a vulnerable organ. There are 50-60 small adrenal branches from 3 main adrenal arteries that form a subcapsular plexus. This plexus drains into the medullary sinusoids through only a few venules. So any cause leading to an increase in the adrenal venous pressure would lead to intraglandular hemorrhage.
There is an increased synthesis of cortisol, including adrenaline, by the adrenal gland in any stressful situation. The increased serum adrenocorticotrophin (ACTH) raises adrenal blood flow, which increases pressure within the vessels. This situation is further accentuated by platelet aggregation in the adrenal veins induced by adrenaline.[22]
Other theories described to explain the hemorrhage include toxin-mediated vasculitis and coagulopathy in association with disseminated intravascular coagulation (DIC). In an experiment done by Levin and Cluff, endotoxin could induce adrenal hemorrhage only when there was an increased activity of the adrenal gland, which suggested that the increased metabolic activity associated with an increase in the production of corticosteroids was needed for the endotoxin to induce hemorrhage in the gland.[23] In the study by Guarner J, et al., various amounts of bacteria and bacterial antigens were seen at the site of hemorrhage in 79% of cases while the remaining 21% cases were not associated with bacterial antigens. However, even among those with antigens, a correlation between the amount of adrenal hemorrhage and the number of bacteria and bacterial antigens could not be established.[13] DIC may lead to venous thrombosis in the adrenal gland with consequent hemorrhage into the gland. But multiple cases of WFS have been reported without accompanying DIC.
Histopathology
On gross examination, hemorrhage is present in the adrenal glands. On microscopic examination, hemorrhage may be visible in all layers of the gland with areas of necrosis using hematoxylin-eosin stain. Microvascular thrombi may also be a feature in histology.
History and Physical
Patients may present suddenly or in the background of ongoing infection with symptoms and signs suggestive of adrenal insufficiency. The principal manifestation of WFS is shock. Patients often have nonspecific symptoms like rapid onset headache, fever, weakness, fatigue, abdominal or flank pain, anorexia, nausea or vomiting, confusion, or disorientation.
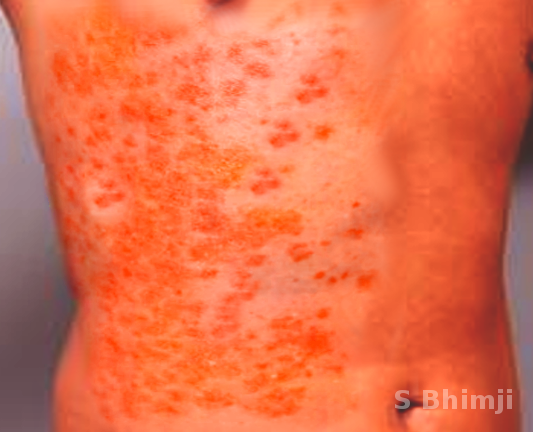
On abdominal examination, rigidity or rebound tenderness may be present. WFS associated with meningococcemia characteristically demonstrates petechial rash, DIC, purpura fulminans along with neurological manifestations seen in meningitis. The petechial rash usually develops on the trunk and lower portions of the body but can develop over mucous membranes, as well. The rash can coalesce to form larger purpura and ecchymoses. The petechiae usually relate to the degree of thrombocytopenia. Thus the clinician must be vigilant about these rashes as they can be of value in anticipating bleeding complications due to DIC.
It is challenging to diagnose WFS, especially in the setting of ongoing sepsis, which may masquerade as septic shock. Surprisingly hypotension precedes shock only in approximately half of all patients.[24]
Evaluation
When Waterhouse Friderichsen syndrome is suspected, complete blood work is required. Fall in hemoglobin and hematocrit levels suggest some form of occult bleeding. Leukocytosis may be present because of underlying bacterial infection. Low mineralocorticoid levels lead to hyponatremia and hyperkalemia. Hyponatremia also occurs due to the Syndrome of inappropriate diuretic hormone (SIADH) caused by cortisol deficiency. Volume contraction with accompanying prerenal azotemia may also be a feature. However, the absence of these electrolyte derangements should not exclude the diagnosis. Hypoglycemia, attributed to decreased glucocorticoid, tends to occur in WFS but is not severe, and it is readily correctable. Arterial Blood Gas analysis reveals metabolic acidosis.
Levels of plasma adrenocorticotrophic hormone (ACTH), cortisol, aldosterone, and renin activity should be obtained to assess adrenal function. There is a decrease in cortisol level with a rise in ACTH level, a decrease in aldosterone level, and a rise in renin level, features consistent with primary adrenal insufficiency. If the diagnosis remains in doubt, one may administer cosyntropin (ACTH) to assess the adrenal function. CT scan is often used to evaluate adrenal hemorrhage in stable patients. Unstable patients may require an ultrasound at the bedside. Electrocardiography is also necessary to assess any changes in rhythm secondary to hyperkalemia.
Treatment / Management
Patients with Waterhouse Friderichsen syndrome present with sepsis. A blood sample should be drawn, and treatment started immediately before obtaining the results. Management includes supportive therapy for sepsis with volume resuscitation, appropriate antibiotic coverage, vasopressor to ensure end-organ perfusion, and other supportive care. A bolus of D5 NS (5% dextrose with 0.9 % normal saline) 20 ml/kg is given over 1 hour so that underlying hypoglycemia is also corrected. If shock is persistent, repeat NS up to a total of 60 ml/kg in 1 hour. Hypoglycemia is treatable by giving 2 to 4 ml/kg of 25% dextrose as a bolus. Steroid replacement is in the form of hydrocortisone, given in the dose of 50 to 100 mg/m^2 as a bolus. The dose of hydrocortisone based on age is 25 mg for children up to 3 years, 50 mg for those from 3 to 12 years, and 100 mg for 12 years and older. The same dose of steroid as a bolus is given over 24 hours, either continuously or in four divided doses.
Mineralocorticoid replacement is not needed acutely because it takes a long time to show its sodium-retaining effects. The decreased production of antidiuretic hormone due to hydrocortisone administration along with normal saline infusion helps correct hyponatremia. In most instances, hyperkalemia improves with fluids and hydrocortisone. Rarely, one may need to administer insulin and glucose, especially if hyperkalemia is severe and symptomatic. The monitoring of electrolytes and water balance is essential throughout the treatment. Once the crisis is well controlled, the maintenance dose of glucocorticoid and mineralocorticoid gets administered regularly.
Conservative management is the recommended approach in cases of traumatic adrenal hemorrhage in the absence of ongoing bleeding.[25] Conservative management includes supportive care, hematocrit monitoring, and blood transfusion as needed. In non-operative management, follow-up imaging is essential to assess the resolution of adrenal hematoma. This imaging also helps in differentiating the benign adrenal hematoma from any adrenal mass that would require surgery.
Operative management in the form of angioembolization of one of the vessels supplying the adrenal gland may be necessary to control the ongoing bleeding.[26] In a study by Mehrazin, surgical exploration was carried out in 3.8 % of cases of traumatic adrenal hemorrhage. Among those requiring surgical exploration, the rate of adrenalectomy was 3.1%.[27]
Differential Diagnosis
The diagnosis of Waterhouse Friderichsen syndrome requires a high degree of suspicion. With the ongoing bacterial infection, it is highly likely to be confused with septic shock. WFS should be mainly in mind if the shock does not respond to intravenous fluids and vasopressors. Hypotension with low body fluid, hyponatremia, and prerenal azotemia are also features of hypovolemia. So WFS may be confused with hypovolemic shock, especially in the absence of elements of bacterial infection and vomiting as a predominant symptom.
Other conditions presenting as an adrenal crisis merit consideration as well. In neonates, congenital adrenal hyperplasia due to 21-hydroxylase deficiency may present as an adrenal crisis within the first few days to weeks of life. Infants with obstructive uropathy, pyelonephritis, or tubulointerstitial nephritis may also present with salt-losing crises with vomiting, hyponatremia, and hyperkalemia.[28]
Prognosis
The prognosis of Waterhouse-Friderichsen syndrome varies according to the severity of illness. Approximately 15 % of patients with significant acute bilateral adrenal bleeding have a fatal outcome. The case fatality rate is almost 50 % in cases of delay in diagnosis and appropriate treatment.
Although the mortality is high with bilateral adrenal hemorrhage, patients do recover with appropriate management on time. Those who recover need treatment with mineralocorticoid and glucocorticoid depending on their electrolyte status and response to the treatment. Although the recovery is unpredictable, research has also shown that those with AH can regain some degree of adrenal function after the acute presentation.[19] There are no randomized clinical studies to tell the duration of the treatment exactly. So, close follow-up and re-evaluation are necessary. A few long term follow-up studies have shown that recovery may be possible in some cases that no longer require glucocorticoid and mineralocorticoid replacement.[29]
Pearls and Other Issues
Despite aggressive treatment, this disorder carries a very high mortality.
Enhancing Healthcare Team Outcomes
Waterhouse Friderichsen syndrome is a rare condition that presents with nonspecific symptoms and requires the efforts of an interprofessional healthcare team. In those patients with a risk factor for adrenal hemorrhage, clinicians should keep their mind broad and open, especially when the hypotension does not respond to vasopressor alone. The management involves interprofessional care that includes hospitalist, intensivist, endocrinologist, and pharmacist.
The patient monitoring is usually done by the ICU nurses who should be aware of the syndrome and its presentation. The pharmacist should ensure that the patient is on the appropriate antibiotics in cases associated with sepsis. Also, the pharmacist must ensure that the patient is receiving glucocorticoids, working with nursing and the clinician team on dosing and administration. Close communication between the interprofessional team is vital to improving patient outcomes. [Level 5]