Continuing Education Activity
Pemphigus is a broad term used to describe a rare group of bullous autoimmune diseases that affect the skin and mucosal membranes. The hallmark presentation of pemphigus is the presence of widespread flaccid blisters and erosions erupting on the skin and oral mucosa. Drug-induced pemphigus is caused by a combination of biochemical interactions and aberrant stimulation of host B cells producing intracellular IgG antibodies. These autoantibodies attack the desmogleins, causing the cells within the epidermis to separate, a process called acantholysis. While there are several factors known to trigger the disease, drugs continue to be the leading cause of pemphigus. This activity reviews the etiology, presentation, evaluation, and management of drug-induced pemphigus, including inciting agents and reviews the role of the interprofessional team in evaluating, diagnosing, and managing the condition.
Objectives:
Describe the patient presentation of drug-induced pemphigus in a patient.
Review the typical drug class offenders that most commonly contribute to drug-induced pemphigus.
Discuss the treatment and management strategies to address drug-induced pemphigus.
Describe the possible interprofessional team strategies for improving care coordination and communication to advance the evaluation and treatment of drug-induced pemphigus and improve outcomes.
Introduction
Pemphigus is a broad term used to describe a rare group of bullous autoimmune diseases that affect the skin and mucosal membranes. The hallmark presentation of pemphigus is the presence of widespread flaccid blisters and erosions erupting on the skin and oral mucosa. Drug-induced pemphigus is caused by a combination of biochemical interactions and aberrant stimulation of host B cells producing intracellular IgG antibodies. These autoantibodies attack the desmogleins, causing the cells within the epidermis to separate, a process called acantholysis. While there are several factors known to trigger the disease, drugs continue to be the leading cause of pemphigus. [1][2][3]
Etiology
Many known triggers have been linked to pemphigus, but drugs continue to be the most prevalent cause of the disease. The inciting medications can be classified based on their chemical structure, with the main groups being thiols drugs, phenol drugs, and non-thiol/phenol drugs. Thiol drugs contain a sulfhydryl (-SH) group in their chemical structure and have been known to be the most common cause of pemphigus. Some of the most noteworthy thiols reported as triggering pemphigus are penicillamine, captopril, and tiopronine. The literature suggests thiol drugs promote acantholysis by stimulating enzymes like plasminogen activator, which disaggregate keratinocytes and inhibiting enzymes that promote keratinocyte aggregation.
Phenol drugs disrupt the integrity of cellular adherence mechanisms by stimulating keratinocytes to release proinflammatory cytokines. The release of tumor necrosis factor-alpha and interleukin-1 from cells drive complement and protease activation, which contribute to acantholysis. The most noteworthy phenols include aspirin, heroin, rifampin, and levodopa.
Many non-thiol and non-phenol drugs have also been classically described in pemphigus. These agents may cause acantholysis through alternative pathways such as the activation of autoantibodies or altering the target antigen structure on keratinocytes. Examples of these agents include non-steroid, anti-inflammatory drugs, and calcium-channel blockers.[4][5][6]
Epidemiology
The incidence of pemphigus is rare and has been estimated to be 1 in 100,000 people. While the disease is likely multifactorial, drug-induced pemphigus is the most common cause. Pemphigus vulgaris is the most common variant, with an incidence as high as 0.1 to 0.5 per 100,000 people. Pemphigus vulgaris also has a higher incidence among the Jewish population and in those individuals of Mediterranean descent. There seems to be an equal incidence in both males and females with an average age of 40 to 60 years. The most common offending drugs include D-penicillamine, captopril, and penicillin. One study reported an incidence as high as 7% among individuals taking penicillamine for the last six months.[7]
Pathophysiology
Drug-induced pemphigus is likely both a biochemical and immunologic process. The chemical structure of certain inciting agents has been found to contribute to the cell-to-cell dyshesion. Intracellular antibodies have also been demonstrated in drug-induced pemphigus.
Thiol drugs, the most common pemphigus-inducing agents, have been shown to promote acantholysis by chemically interfering with the keratinocyte membrane. The binding of drugs with the thiol group can cause a disulfide bond to form, making the cell-to-cell adhesion not possible, resulting in acantholysis. Other proposed mechanisms for biochemical acantholysis include activation of proteolytic enzymes and the production of plasmin. Both penicillamine and captopril have been shown to impair the activation of plasminogen activator inhibitors.
Medications also induce pemphigus through immunologic acantholysis via the formation of IgG autoantibody production against desmoglein 1 and desmoglein 3. Desmoglein is the major glycoprotein component of cadherins that help make up the structural elements of desmosomes. Desmosomes help attach adjacent keratinocytes.[8]
Histopathology
Biopsies of the bulla or erosions, seen in drug-induced pemphigus vulgaris, can be visualized under light microscopy to show the separation of keratinocytes above the basal cell layer of the epidermis. Vesicles may also be seen containing separated keratinocytes in the suprabasal epidermis. In pemphigus foliaceus, the skin lesions are more superficial, and acantholysis and vesicle formation are seen in the granular layer of the epidermis. Direct immunofluorescence staining may reveal IgG antibodies to the Dsg1 and Dsg3 on the intercellular and transmembrane domains of keratinocytes within the epidermis. Analysis of the patient's serum using ELISA technique may also be used to identify IgG autoantibodies to the target antigens Dsg1 and/or Dsg3, which are present in the desmosomes of keratinocytes.
History and Physical
Drug-induced pemphigus vulgaris and pemphigus foliaceus are most frequently associated with the use of d-penicillamine. A thorough history consisting of medications and exposure time is important to help identify the offending agent. Identification of flaccid or erosion lesions should include inspection of oral and vaginal mucosa and a full body scan of the skin.
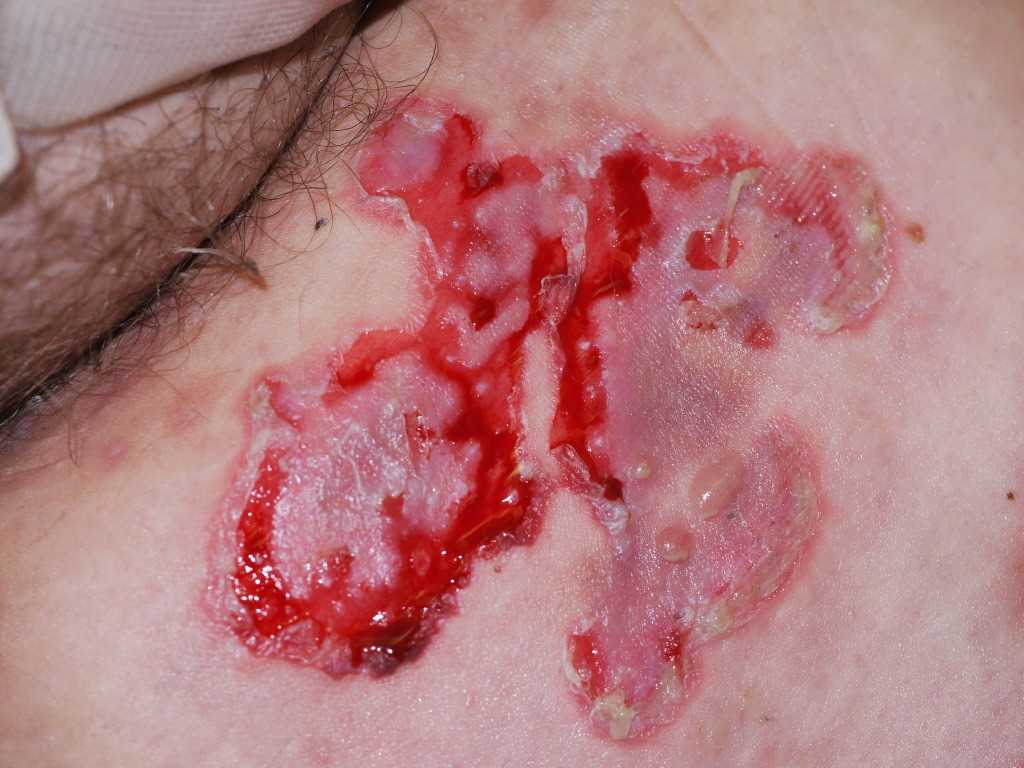
Pemphigus vulgaris traditionally presents with flaccid, easily ruptured bulla on the surface of oral mucosa and skin. The fluid-filled vesicles and bulla are serous-filled and are easily ruptured. Small solitary vesicles appear initially on the scalp, face, chest, axillae, groin, or back. The lesions can progress to a widespread confluence of flaccid blisters and erosions. Intact bullae are rarely seen in the erosions of the oropharynx, larynx, or vagina. The lesions are non-pruritic but can be very painful as the blisters can rupture and erode the superficial skin.
Pemphigus foliaceous typically has no mucosal involvement and begins with scaly crusted lesions with an erythematous base. Initial solitary well-demarcated lesions may appear on seborrheic regions of the face, scalp, chest, back, and abdomen and can progress to a generalized exfoliative disease.
There is a positive Nikolsky sign with pemphigus where gentle pressure with a finger on the patient's affected skin can produce a disruption of the superficial skin layer. This differentiates tense bullous lesions seen in pemphigoid, which do not rupture with the slight pressure of a finger.
Evaluation
While the majority of pemphigus cases are diagnosed clinically, a skin biopsy and serum analysis can also confirm the disease. Pemphigus patients will have a history of painful superficial flaccid bulla or erosions on the skin or mucosa following drug exposure. A skin biopsy can be analyzed by light microscopy showing the separation of keratinocytes above the basal cell layer. Anti-desmoglein 1 and 3 autoantibodies can be evaluated using the indirect immunofluorescent staining or ELISA.[7]
Treatment / Management
The mainstay of treatment involves the cessation of the causal agent and the use of immunosuppressants or immunomodulators to turn off the host autoimmune response. Pemphigus can be life-threatening and requires intensive and prompt use of systemic corticosteroids for induction at a dose of 1mg/kg body weight. Immunosuppressive agents such as azathioprine, mycophenolate mofetil, methotrexate, or cyclophosphamide are also used in severe disease. The use of an anti-CD-20 antibody, rituximab, has also shown to be a promising therapy for refractory pemphigus in a number of cases by targeting the aberrant B cells. Some cases also suggest an exacerbation of disease after the treatment with rituximab. Overall, there is a lack of well-controlled clinical studies on the proper treatment of drug-induced pemphigus, but the mainstay of the therapy continues to consist of a combination of corticosteroids and an immunosuppressive agent such as azathioprine. [9]
Differential Diagnosis
There are a variety of autoimmune bullous diseases that disrupt the cellular adhesion proteins in the skin and mucous membranes. These lesions can be differentiated by the location of skin lesions, mucous membrane involvement, distribution of lesions, histopathology, and immunopathology, as well as serum antibody assays.
- Pemphigus vulgaris
- Pemphigus vegetans
- Pemphigus foliaceus
- Bullous pemphigoid
- Herpes gestationis
- Mucous membrane pemphigoid
- Epidermolysis bullosa acquisita
- Linear IgA dermatosis
- Bullous lupus
- Dermatitis herpetiformis
Prognosis
The prognosis is good in patients, with thiol-drug-induced pemphigus, who lack cell surface autoantibodies. Once the thiol-drug is discontinued, at least 50% of patients will see an improvement. Unfortunately, a significant number of patients with drug-induced pemphigus also have cell surface autoantibodies, and these individuals will have a chronic course similar to pemphigus vulgaris. While mortality rates for drug-induced pemphigus are not known, isolated deaths have been reported. However, when chronic pemphigus persists, it carries very high morbidity and a poor quality of life. Patients often develop extensive skin lesions marked by burning sensations and pain. When the oral cavity is involved, it can lead to decreased oral intake, dehydration, and significant weight loss. [1][3] [Level 5]
Complications
- Secondary bacterial infections
- Skin ulcers
- Sepsis
- Gangrene
- Severe pain
- Poor cosmesis
Pearls and Other Issues
The identification of the specific drug driving the acantholytic reaction behind pemphigus can be difficult. Patients are often exposed to a variety of medications that can induce pemphigus throughout their clinical course. There can also be a latency period between the use of the causative agent and the disease onset. These factors often make the detection of the disease-causing drug challenging.
Enhancing Healthcare Team Outcomes
Pemphigus is a serious skin disorder with very high morbidity. The role of the pharmacist is critical. When dispensing any thiol-related medication, the risk of drug-induced pemphigus must be considered. The sooner the drug is discontinued, the better the prognosis. Once the drug-induced pemphigus has developed, besides discontinuing the drug, the nurse or physician should educate the patient on a recommended diet. Certain foods contain phenols and thiols that can exacerbate the condition. Thus a dietary consult is necessary. Foods that contain phenol and thiol-like compounds include chives, garlic, onion, black pepper, cashew, and mangoes. For patients who develop burning pain, the pharmacist should encourage compliance with medical treatments and review how the medication is taken and possible side effects. Follow up of these patients is critical as many of them will require potent immunosuppressive drugs for symptom relief. [10][11] [Level 3] Nurses often facilitate follow and referrals. [Level 5]