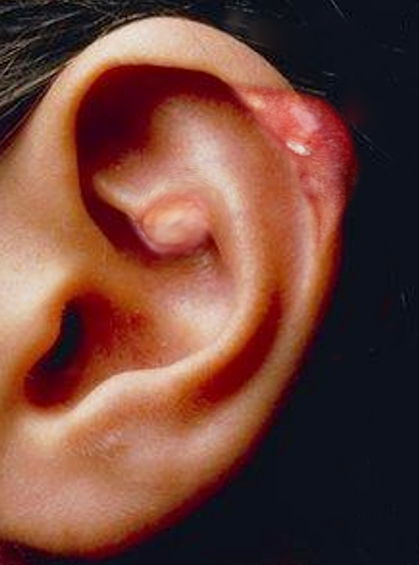
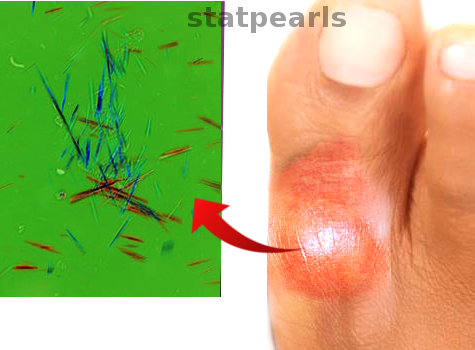
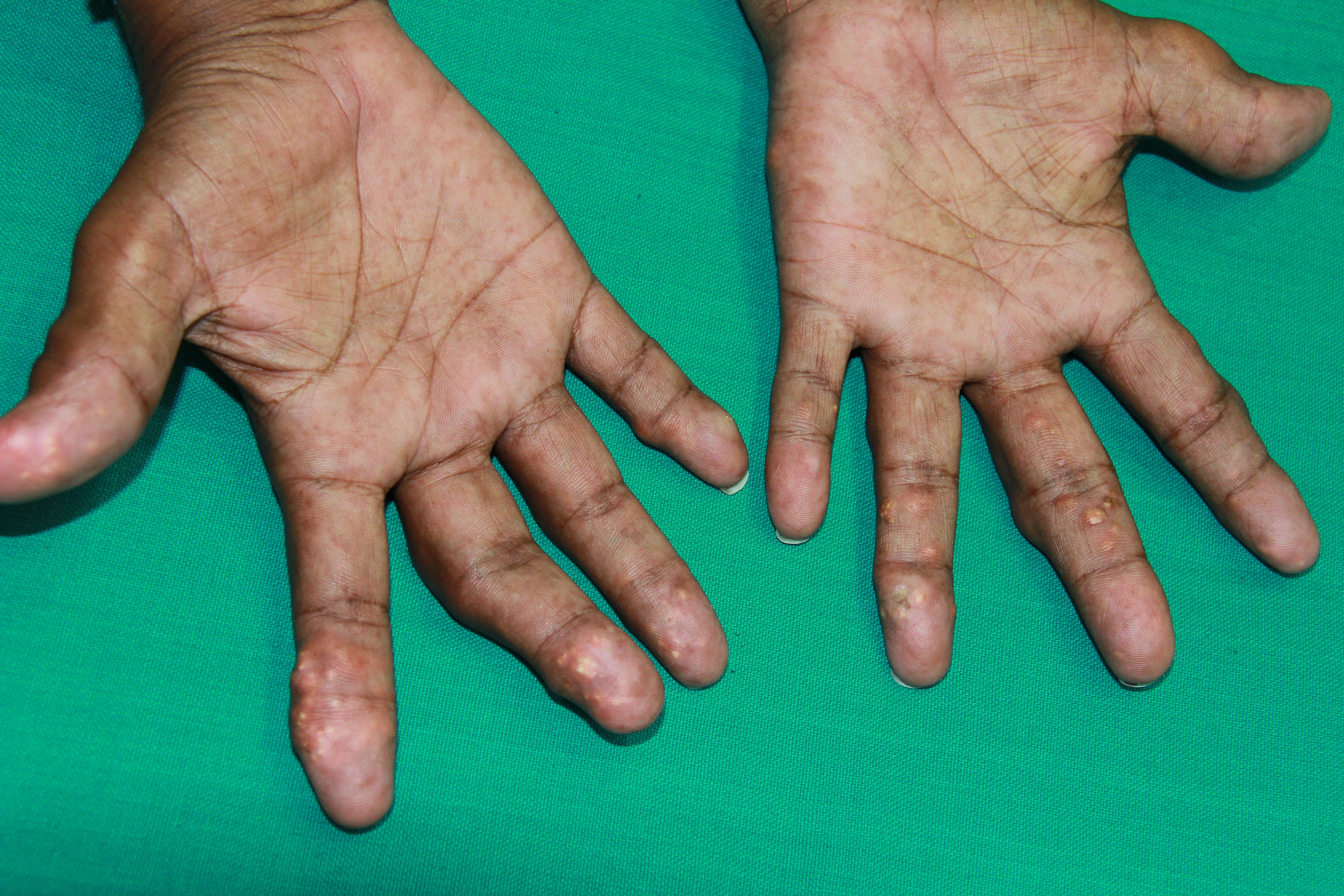
[1]
Neogi T. Gout. Annals of internal medicine. 2016 Jul 5:165(1):ITC1-ITC16. doi: 10.7326/AITC201607050. Epub
[PubMed PMID: 27380294]
[2]
Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet (London, England). 2016 Oct 22:388(10055):2039-2052. doi: 10.1016/S0140-6736(16)00346-9. Epub 2016 Apr 21
[PubMed PMID: 27112094]
[3]
Richette P, Bardin T. Gout. Lancet (London, England). 2010 Jan 23:375(9711):318-28. doi: 10.1016/S0140-6736(09)60883-7. Epub 2009 Aug 17
[PubMed PMID: 19692116]
[4]
Nuki G, Simkin PA. A concise history of gout and hyperuricemia and their treatment. Arthritis research & therapy. 2006:8 Suppl 1(Suppl 1):S1
[PubMed PMID: 16820040]
[5]
Loeb JN. The influence of temperature on the solubility of monosodium urate. Arthritis and rheumatism. 1972 Mar-Apr:15(2):189-92
[PubMed PMID: 5027604]
[6]
Nian YL, You CG. Susceptibility genes of hyperuricemia and gout. Hereditas. 2022 Aug 4:159(1):30. doi: 10.1186/s41065-022-00243-y. Epub 2022 Aug 4
[PubMed PMID: 35922835]
[7]
Merriman TR, Choi HK, Dalbeth N. The genetic basis of gout. Rheumatic diseases clinics of North America. 2014 May:40(2):279-90. doi: 10.1016/j.rdc.2014.01.009. Epub 2014 Feb 19
[PubMed PMID: 24703347]
[8]
Choi HK, Mount DB, Reginato AM, American College of Physicians, American Physiological Society. Pathogenesis of gout. Annals of internal medicine. 2005 Oct 4:143(7):499-516
[PubMed PMID: 16204163]
[9]
Tan PK, Farrar JE, Gaucher EA, Miner JN. Coevolution of URAT1 and Uricase during Primate Evolution: Implications for Serum Urate Homeostasis and Gout. Molecular biology and evolution. 2016 Sep:33(9):2193-200. doi: 10.1093/molbev/msw116. Epub 2016 Jun 26
[PubMed PMID: 27352852]
Level 3 (low-level) evidence
[10]
Abhishek A, Roddy E, Doherty M. Gout - a guide for the general and acute physicians. Clinical medicine (London, England). 2017 Feb:17(1):54-59. doi: 10.7861/clinmedicine.17-1-54. Epub
[PubMed PMID: 28148582]
[11]
Scott JT. Asymptomatic hyperuricaemia. British medical journal (Clinical research ed.). 1987 Apr 18:294(6578):987-8
[PubMed PMID: 3119013]
[12]
Dalbeth N, Phipps-Green A, Frampton C, Neogi T, Taylor WJ, Merriman TR. Relationship between serum urate concentration and clinically evident incident gout: an individual participant data analysis. Annals of the rheumatic diseases. 2018 Jul:77(7):1048-1052. doi: 10.1136/annrheumdis-2017-212288. Epub 2018 Feb 20
[PubMed PMID: 29463518]
[13]
Wu EQ, Patel PA, Mody RR, Yu AP, Cahill KE, Tang J, Krishnan E. Frequency, risk, and cost of gout-related episodes among the elderly: does serum uric acid level matter? The Journal of rheumatology. 2009 May:36(5):1032-40. doi: 10.3899/jrheum.080487. Epub 2009 Apr 15
[PubMed PMID: 19369467]
[14]
Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ (Clinical research ed.). 2008 Feb 9:336(7639):309-12. doi: 10.1136/bmj.39449.819271.BE. Epub 2008 Jan 31
[PubMed PMID: 18244959]
[15]
Choi HK, Willett W, Curhan G. Fructose-rich beverages and risk of gout in women. JAMA. 2010 Nov 24:304(20):2270-8. doi: 10.1001/jama.2010.1638. Epub 2010 Nov 10
[PubMed PMID: 21068145]
[16]
Dalbeth N, So A. Hyperuricaemia and gout: state of the art and future perspectives. Annals of the rheumatic diseases. 2010 Oct:69(10):1738-43. doi: 10.1136/ard.2010.136218. Epub
[PubMed PMID: 20858623]
Level 3 (low-level) evidence
[17]
Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. The New England journal of medicine. 2004 Mar 11:350(11):1093-103
[PubMed PMID: 15014182]
[18]
Juraschek SP, Yokose C, McCormick N, Miller ER 3rd, Appel LJ, Choi HK. Effects of Dietary Patterns on Serum Urate: Results From a Randomized Trial of the Effects of Diet on Hypertension. Arthritis & rheumatology (Hoboken, N.J.). 2021 Jun:73(6):1014-1020. doi: 10.1002/art.41614. Epub 2021 Apr 23
[PubMed PMID: 33615722]
Level 1 (high-level) evidence
[19]
Rai SK, Fung TT, Lu N, Keller SF, Curhan GC, Choi HK. The Dietary Approaches to Stop Hypertension (DASH) diet, Western diet, and risk of gout in men: prospective cohort study. BMJ (Clinical research ed.). 2017 May 9:357():j1794. doi: 10.1136/bmj.j1794. Epub 2017 May 9
[PubMed PMID: 28487277]
[20]
Huang HY, Appel LJ, Choi MJ, Gelber AC, Charleston J, Norkus EP, Miller ER 3rd. The effects of vitamin C supplementation on serum concentrations of uric acid: results of a randomized controlled trial. Arthritis and rheumatism. 2005 Jun:52(6):1843-7
[PubMed PMID: 15934094]
Level 1 (high-level) evidence
[21]
Gao X, Curhan G, Forman JP, Ascherio A, Choi HK. Vitamin C intake and serum uric acid concentration in men. The Journal of rheumatology. 2008 Sep:35(9):1853-8
[PubMed PMID: 18464304]
[22]
Juraschek SP, Miller ER 3rd, Gelber AC. Effect of oral vitamin C supplementation on serum uric acid: a meta-analysis of randomized controlled trials. Arthritis care & research. 2011 Sep:63(9):1295-306. doi: 10.1002/acr.20519. Epub
[PubMed PMID: 21671418]
Level 1 (high-level) evidence
[23]
Choi HK, Gao X, Curhan G. Vitamin C intake and the risk of gout in men: a prospective study. Archives of internal medicine. 2009 Mar 9:169(5):502-7. doi: 10.1001/archinternmed.2008.606. Epub
[PubMed PMID: 19273781]
[24]
Juraschek SP, Gaziano JM, Glynn RJ, Gomelskaya N, Bubes VY, Buring JE, Shmerling RH, Sesso HD. Effects of vitamin C supplementation on gout risk: results from the Physicians' Health Study II trial. The American journal of clinical nutrition. 2022 Sep 2:116(3):812-819. doi: 10.1093/ajcn/nqac140. Epub
[PubMed PMID: 35575611]
[25]
Jacob RA, Spinozzi GM, Simon VA, Kelley DS, Prior RL, Hess-Pierce B, Kader AA. Consumption of cherries lowers plasma urate in healthy women. The Journal of nutrition. 2003 Jun:133(6):1826-9
[PubMed PMID: 12771324]
[26]
Zhang Y, Neogi T, Chen C, Chaisson C, Hunter DJ, Choi HK. Cherry consumption and decreased risk of recurrent gout attacks. Arthritis and rheumatism. 2012 Dec:64(12):4004-11. doi: 10.1002/art.34677. Epub
[PubMed PMID: 23023818]
[27]
Glynn RJ, Campion EW, Silbert JE. Trends in serum uric acid levels 1961--1980. Arthritis and rheumatism. 1983 Jan:26(1):87-93
[PubMed PMID: 6824508]
[28]
Takiue Y, Hosoyamada M, Kimura M, Saito H. The effect of female hormones upon urate transport systems in the mouse kidney. Nucleosides, nucleotides & nucleic acids. 2011 Feb:30(2):113-9. doi: 10.1080/15257770.2010.551645. Epub
[PubMed PMID: 21360409]
[29]
Rho YH, Zhu Y, Choi HK. The epidemiology of uric acid and fructose. Seminars in nephrology. 2011 Sep:31(5):410-9. doi: 10.1016/j.semnephrol.2011.08.004. Epub
[PubMed PMID: 22000647]
[30]
Hak AE, Choi HK. Menopause, postmenopausal hormone use and serum uric acid levels in US women--the Third National Health and Nutrition Examination Survey. Arthritis research & therapy. 2008:10(5):R116. doi: 10.1186/ar2519. Epub 2008 Sep 26
[PubMed PMID: 18822120]
Level 3 (low-level) evidence
[31]
Arromdee E, Michet CJ, Crowson CS, O'Fallon WM, Gabriel SE. Epidemiology of gout: is the incidence rising? The Journal of rheumatology. 2002 Nov:29(11):2403-6
[PubMed PMID: 12415600]
[32]
Choi H. Epidemiology of crystal arthropathy. Rheumatic diseases clinics of North America. 2006 May:32(2):255-73, v
[PubMed PMID: 16716879]
[33]
Ragab G, Elshahaly M, Bardin T. Gout: An old disease in new perspective - A review. Journal of advanced research. 2017 Sep:8(5):495-511. doi: 10.1016/j.jare.2017.04.008. Epub 2017 May 10
[PubMed PMID: 28748116]
Level 3 (low-level) evidence
[34]
Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, Jordan JM, Katz JN, Kremers HM, Wolfe F, National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis and rheumatism. 2008 Jan:58(1):26-35. doi: 10.1002/art.23176. Epub
[PubMed PMID: 18163497]
[35]
Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis and rheumatism. 2011 Oct:63(10):3136-41. doi: 10.1002/art.30520. Epub
[PubMed PMID: 21800283]
Level 3 (low-level) evidence
[36]
Kramer HM, Curhan G. The association between gout and nephrolithiasis: the National Health and Nutrition Examination Survey III, 1988-1994. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2002 Jul:40(1):37-42
[PubMed PMID: 12087559]
Level 3 (low-level) evidence
[37]
McCormick N, Lu N, Yokose C, Joshi AD, Sheehy S, Rosenberg L, Warner ET, Dalbeth N, Merriman TR, Saag KG, Zhang Y, Choi HK. Racial and Sex Disparities in Gout Prevalence Among US Adults. JAMA network open. 2022 Aug 1:5(8):e2226804. doi: 10.1001/jamanetworkopen.2022.26804. Epub 2022 Aug 1
[PubMed PMID: 35969396]
[38]
Elfishawi MM, Zleik N, Kvrgic Z, Michet CJ Jr, Crowson CS, Matteson EL, Bongartz T. The Rising Incidence of Gout and the Increasing Burden of Comorbidities: A Population-based Study over 20 Years. The Journal of rheumatology. 2018 Apr:45(4):574-579. doi: 10.3899/jrheum.170806. Epub 2017 Dec 15
[PubMed PMID: 29247151]
[39]
Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M. Rising burden of gout in the UK but continuing suboptimal management: a nationwide population study. Annals of the rheumatic diseases. 2015 Apr:74(4):661-7. doi: 10.1136/annrheumdis-2013-204463. Epub 2014 Jan 15
[PubMed PMID: 24431399]
[40]
Bai L, Zhou JB, Zhou T, Newson RB, Cardoso MA. Incident gout and weight change patterns: a retrospective cohort study of US adults. Arthritis research & therapy. 2021 Mar 2:23(1):69. doi: 10.1186/s13075-021-02461-7. Epub 2021 Mar 2
[PubMed PMID: 33653403]
Level 2 (mid-level) evidence
[41]
Hwang J, Lee MY, Ahn JK, Cha HS. Relationship Between Changing Body Mass Index and Serum Uric Acid Alteration Among Clinically Apparently Healthy Korean Men. Arthritis care & research. 2022 Aug:74(8):1277-1286. doi: 10.1002/acr.24576. Epub 2022 Apr 30
[PubMed PMID: 33544980]
[42]
Liu K, Yao Y, Chen W, Mao Y, Ye D, Wen C. Modifiable risk factors and incidence of gout: Estimation of population attributable fraction in the US. Seminars in arthritis and rheumatism. 2022 Aug:55():152040. doi: 10.1016/j.semarthrit.2022.152040. Epub 2022 Jun 3
[PubMed PMID: 35679791]
[43]
Annemans L, Spaepen E, Gaskin M, Bonnemaire M, Malier V, Gilbert T, Nuki G. Gout in the UK and Germany: prevalence, comorbidities and management in general practice 2000-2005. Annals of the rheumatic diseases. 2008 Jul:67(7):960-6
[PubMed PMID: 17981913]
[44]
Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M. Comorbidities in patients with gout prior to and following diagnosis: case-control study. Annals of the rheumatic diseases. 2016 Jan:75(1):210-7. doi: 10.1136/annrheumdis-2014-206410. Epub 2014 Nov 14
[PubMed PMID: 25398375]
Level 2 (mid-level) evidence
[45]
Krishnan E. Chronic kidney disease and the risk of incident gout among middle-aged men: a seven-year prospective observational study. Arthritis and rheumatism. 2013 Dec:65(12):3271-8. doi: 10.1002/art.38171. Epub
[PubMed PMID: 23982888]
Level 2 (mid-level) evidence
[46]
Moon KW, Kim MJ, Choi IA, Shin K. Cardiovascular Risks in Korean Patients with Gout: Analysis Using a National Health Insurance Service Database. Journal of clinical medicine. 2022 Apr 11:11(8):. doi: 10.3390/jcm11082124. Epub 2022 Apr 11
[PubMed PMID: 35456221]
[47]
Cipolletta E, Tata LJ, Nakafero G, Avery AJ, Mamas MA, Abhishek A. Association Between Gout Flare and Subsequent Cardiovascular Events Among Patients With Gout. JAMA. 2022 Aug 2:328(5):440-450. doi: 10.1001/jama.2022.11390. Epub
[PubMed PMID: 35916846]
[48]
Disveld IJM, Zoakman S, Jansen TLTA, Rongen GA, Kienhorst LBE, Janssens HJEM, Fransen J, Janssen M. Crystal-proven gout patients have an increased mortality due to cardiovascular diseases, cancer, and infectious diseases especially when having tophi and/or high serum uric acid levels: a prospective cohort study. Clinical rheumatology. 2019 May:38(5):1385-1391. doi: 10.1007/s10067-019-04520-6. Epub 2019 Mar 30
[PubMed PMID: 30929152]
[49]
Ioachimescu AG, Brennan DM, Hoar BM, Hazen SL, Hoogwerf BJ. Serum uric acid is an independent predictor of all-cause mortality in patients at high risk of cardiovascular disease: a preventive cardiology information system (PreCIS) database cohort study. Arthritis and rheumatism. 2008 Feb:58(2):623-30. doi: 10.1002/art.23121. Epub
[PubMed PMID: 18240236]
[50]
Vargas-Santos AB, Neogi T, da Rocha Castelar-Pinheiro G, Kapetanovic MC, Turkiewicz A. Cause-Specific Mortality in Gout: Novel Findings of Elevated Risk of Non-Cardiovascular-Related Deaths. Arthritis & rheumatology (Hoboken, N.J.). 2019 Nov:71(11):1935-1942. doi: 10.1002/art.41008. Epub 2019 Sep 25
[PubMed PMID: 31169353]
[51]
Hong JY, Lan TY, Tang GJ, Tang CH, Chen TJ, Lin HY. Gout and the risk of dementia: a nationwide population-based cohort study. Arthritis research & therapy. 2015 May 28:17(1):139. doi: 10.1186/s13075-015-0642-1. Epub 2015 May 28
[PubMed PMID: 26018424]
[52]
Min KH, Kang SO, Oh SJ, Han JM, Lee KE. Association Between Gout and Dementia in the Elderly: A Nationwide Population-Based Cohort Study. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2021 Dec:29(12):1177-1185. doi: 10.1016/j.jagp.2021.01.016. Epub 2021 Jan 30
[PubMed PMID: 33593591]
[53]
Pan SY, Cheng RJ, Xia ZJ, Zhang QP, Liu Y. Risk of dementia in gout and hyperuricaemia: a meta-analysis of cohort studies. BMJ open. 2021 Jun 22:11(6):e041680. doi: 10.1136/bmjopen-2020-041680. Epub 2021 Jun 22
[PubMed PMID: 34158290]
Level 1 (high-level) evidence
[54]
Kim JH, Yim DH, Choi IA, Lee J, Park H, Eom SY. Impact of Clinical Association Between Gout and Dementia: A Nationwide Population-Based Cohort Study in Korea. Arthritis care & research. 2023 May:75(5):1088-1094. doi: 10.1002/acr.24959. Epub 2022 Dec 18
[PubMed PMID: 35604886]
[55]
Lu N, Dubreuil M, Zhang Y, Neogi T, Rai SK, Ascherio A, Hernán MA, Choi HK. Gout and the risk of Alzheimer's disease: a population-based, BMI-matched cohort study. Annals of the rheumatic diseases. 2016 Mar:75(3):547-51. doi: 10.1136/annrheumdis-2014-206917. Epub 2015 Mar 4
[PubMed PMID: 25739830]
[56]
Singh JA, Cleveland JD. Gout and dementia in the elderly: a cohort study of Medicare claims. BMC geriatrics. 2018 Nov 14:18(1):281. doi: 10.1186/s12877-018-0975-0. Epub 2018 Nov 14
[PubMed PMID: 30428833]
[57]
Latourte A, Soumaré A, Bardin T, Perez-Ruiz F, Debette S, Richette P. Uric acid and incident dementia over 12 years of follow-up: a population-based cohort study. Annals of the rheumatic diseases. 2018 Mar:77(3):328-335. doi: 10.1136/annrheumdis-2016-210767. Epub 2017 Jul 28
[PubMed PMID: 28754803]
[58]
Alonso A, Rodríguez LA, Logroscino G, Hernán MA. Gout and risk of Parkinson disease: a prospective study. Neurology. 2007 Oct 23:69(17):1696-700
[PubMed PMID: 17954784]
[59]
De Vera M, Rahman MM, Rankin J, Kopec J, Gao X, Choi H. Gout and the risk of Parkinson's disease: a cohort study. Arthritis and rheumatism. 2008 Nov 15:59(11):1549-54. doi: 10.1002/art.24193. Epub
[PubMed PMID: 18975349]
[60]
Pou MA, Orfila F, Pagonabarraga J, Ferrer-Moret S, Corominas H, Diaz-Torne C. Risk of Parkinson's disease in a gout Mediterranean population: A case-control study. Joint bone spine. 2022 Nov:89(6):105402. doi: 10.1016/j.jbspin.2022.105402. Epub 2022 Apr 30
[PubMed PMID: 35504516]
Level 2 (mid-level) evidence
[61]
Fazlollahi A, Zahmatyar M, Alizadeh H, Noori M, Jafari N, Nejadghaderi SA, Sullman MJM, Gharagozli K, Kolahi AA, Safiri S. Association between gout and the development of Parkinson's disease: a systematic review and meta-analysis. BMC neurology. 2022 Oct 11:22(1):383. doi: 10.1186/s12883-022-02874-0. Epub 2022 Oct 11
[PubMed PMID: 36221048]
Level 1 (high-level) evidence
[62]
Hu LY, Yang AC, Lee SC, You ZH, Tsai SJ, Hu CK, Shen CC. Risk of Parkinson's disease following gout: a population-based retrospective cohort study in Taiwan. BMC neurology. 2020 Sep 8:20(1):338. doi: 10.1186/s12883-020-01916-9. Epub 2020 Sep 8
[PubMed PMID: 32900384]
Level 2 (mid-level) evidence
[63]
Singh JA, Cleveland JD. Gout and the risk of Parkinson's disease in older adults: a study of U.S. Medicare data. BMC neurology. 2019 Jan 5:19(1):4. doi: 10.1186/s12883-018-1234-x. Epub 2019 Jan 5
[PubMed PMID: 30611222]
[64]
Bursill D, Taylor WJ, Terkeltaub R, Abhishek A, So AK, Vargas-Santos AB, Gaffo AL, Rosenthal A, Tausche AK, Reginato A, Manger B, Sciré C, Pineda C, van Durme C, Lin CT, Yin C, Albert DA, Biernat-Kaluza E, Roddy E, Pascual E, Becce F, Perez-Ruiz F, Sivera F, Lioté F, Schett G, Nuki G, Filippou G, McCarthy G, da Rocha Castelar Pinheiro G, Ea HK, Tupinambá HA, Yamanaka H, Choi HK, Mackay J, ODell JR, Vázquez Mellado J, Singh JA, Fitzgerald JD, Jacobsson LTH, Joosten L, Harrold LR, Stamp L, Andrés M, Gutierrez M, Kuwabara M, Dehlin M, Janssen M, Doherty M, Hershfield MS, Pillinger M, Edwards NL, Schlesinger N, Kumar N, Slot O, Ottaviani S, Richette P, MacMullan PA, Chapman PT, Lipsky PE, Robinson P, Khanna PP, Gancheva RN, Grainger R, Johnson RJ, Te Kampe R, Keenan RT, Tedeschi SK, Kim S, Choi SJ, Fields TR, Bardin T, Uhlig T, Jansen T, Merriman T, Pascart T, Neogi T, Klück V, Louthrenoo W, Dalbeth N. Gout, Hyperuricaemia and Crystal-Associated Disease Network (G-CAN) consensus statement regarding labels and definitions of disease states of gout. Annals of the rheumatic diseases. 2019 Nov:78(11):1592-1600. doi: 10.1136/annrheumdis-2019-215933. Epub 2019 Sep 9
[PubMed PMID: 31501138]
Level 3 (low-level) evidence
[65]
Bursill D, Taylor WJ, Terkeltaub R, Kuwabara M, Merriman TR, Grainger R, Pineda C, Louthrenoo W, Edwards NL, Andrés M, Vargas-Santos AB, Roddy E, Pascart T, Lin CT, Perez-Ruiz F, Tedeschi SK, Kim SC, Harrold LR, McCarthy G, Kumar N, Chapman PT, Tausche AK, Vazquez-Mellado J, Gutierrez M, da Rocha Castelar-Pinheiro G, Richette P, Pascual E, Fisher MC, Burgos-Vargas R, Robinson PC, Singh JA, Jansen TL, Saag KG, Slot O, Uhlig T, Solomon DH, Keenan RT, Scire CA, Biernat-Kaluza E, Dehlin M, Nuki G, Schlesinger N, Janssen M, Stamp LK, Sivera F, Reginato AM, Jacobsson L, Lioté F, Ea HK, Rosenthal A, Bardin T, Choi HK, Hershfield MS, Czegley C, Choi SJ, Dalbeth N. Gout, Hyperuricemia, and Crystal-Associated Disease Network Consensus Statement Regarding Labels and Definitions for Disease Elements in Gout. Arthritis care & research. 2019 Mar:71(3):427-434. doi: 10.1002/acr.23607. Epub
[PubMed PMID: 29799677]
Level 3 (low-level) evidence
[66]
Dalbeth N, Gosling AL, Gaffo A, Abhishek A. Gout. Lancet (London, England). 2021 May 15:397(10287):1843-1855. doi: 10.1016/S0140-6736(21)00569-9. Epub 2021 Mar 30
[PubMed PMID: 33798500]
[67]
Desai J, Steiger S, Anders HJ. Molecular Pathophysiology of Gout. Trends in molecular medicine. 2017 Aug:23(8):756-768. doi: 10.1016/j.molmed.2017.06.005. Epub 2017 Jul 18
[PubMed PMID: 28732688]
[68]
Chhana A, Lee G, Dalbeth N. Factors influencing the crystallization of monosodium urate: a systematic literature review. BMC musculoskeletal disorders. 2015 Oct 14:16():296. doi: 10.1186/s12891-015-0762-4. Epub 2015 Oct 14
[PubMed PMID: 26467213]
Level 1 (high-level) evidence
[69]
Pascual E, Addadi L, Andrés M, Sivera F. Mechanisms of crystal formation in gout-a structural approach. Nature reviews. Rheumatology. 2015 Dec:11(12):725-30. doi: 10.1038/nrrheum.2015.125. Epub 2015 Sep 15
[PubMed PMID: 26369610]
[70]
Franklin BS, Mangan MS, Latz E. Crystal Formation in Inflammation. Annual review of immunology. 2016 May 20:34():173-202. doi: 10.1146/annurev-immunol-041015-055539. Epub 2016 Jan 11
[PubMed PMID: 26772211]
[71]
Ginsberg MH, Kozin F. Mechanisms of cellular interaction with monosodium urate crystals. IgG-dependent and IgG-independent platelet stimulation by urate crystals. Arthritis and rheumatism. 1978 Nov-Dec:21(8):896-903
[PubMed PMID: 737013]
[72]
Giclas PC, Ginsberg MH, Cooper NR. Immunoglobulin G independent activation of the classical complement pathway by monosodium urate crystals. The Journal of clinical investigation. 1979 Apr:63(4):759-64
[PubMed PMID: 438335]
[73]
Fields TR, Abramson SB, Weissmann G, Kaplan AP, Ghebrehiwet B. Activation of the alternative pathway of complement by monosodium urate crystals. Clinical immunology and immunopathology. 1983 Feb:26(2):249-57
[PubMed PMID: 6872344]
[74]
So AK, Martinon F. Inflammation in gout: mechanisms and therapeutic targets. Nature reviews. Rheumatology. 2017 Nov:13(11):639-647. doi: 10.1038/nrrheum.2017.155. Epub 2017 Sep 28
[PubMed PMID: 28959043]
[75]
Chen J, Wu M, Yang J, Wang J, Qiao Y, Li X. The Immunological Basis in the Pathogenesis of Gout. Iranian journal of immunology : IJI. 2017 Jun:14(2):90-98
[PubMed PMID: 28630380]
Level 2 (mid-level) evidence
[76]
Lee JH, Kim HS, Lee JH, Yang G, Kim HJ. Natural Products as a Novel Therapeutic Strategy for NLRP3 Inflammasome-Mediated Gout. Frontiers in pharmacology. 2022:13():861399. doi: 10.3389/fphar.2022.861399. Epub 2022 Mar 16
[PubMed PMID: 35370689]
[77]
Chhana A, Dalbeth N. Structural joint damage in gout. Rheumatic diseases clinics of North America. 2014 May:40(2):291-309. doi: 10.1016/j.rdc.2014.01.006. Epub 2014 Feb 20
[PubMed PMID: 24703348]
[78]
Dalbeth N, Clark B, Gregory K, Gamble G, Sheehan T, Doyle A, McQueen FM. Mechanisms of bone erosion in gout: a quantitative analysis using plain radiography and computed tomography. Annals of the rheumatic diseases. 2009 Aug:68(8):1290-5. doi: 10.1136/ard.2008.094201. Epub 2008 Aug 15
[PubMed PMID: 18708415]
[79]
Dalbeth N, Smith T, Nicolson B, Clark B, Callon K, Naot D, Haskard DO, McQueen FM, Reid IR, Cornish J. Enhanced osteoclastogenesis in patients with tophaceous gout: urate crystals promote osteoclast development through interactions with stromal cells. Arthritis and rheumatism. 2008 Jun:58(6):1854-65. doi: 10.1002/art.23488. Epub
[PubMed PMID: 18512794]
[80]
MCCARTY DJ, HOLLANDER JL. Identification of urate crystals in gouty synovial fluid. Annals of internal medicine. 1961 Mar:54():452-60
[PubMed PMID: 13773775]
[81]
Towiwat P, Chhana A, Dalbeth N. The anatomical pathology of gout: a systematic literature review. BMC musculoskeletal disorders. 2019 Apr 1:20(1):140. doi: 10.1186/s12891-019-2519-y. Epub 2019 Apr 1
[PubMed PMID: 30935368]
Level 1 (high-level) evidence
[82]
Hadler NM, Franck WA, Bress NM, Robinson DR. Acute polyarticular gout. The American journal of medicine. 1974 May:56(5):715-9
[PubMed PMID: 4825606]
[83]
Komarla A, Schumacher R, Merkel PA. Spinal gout presenting as acute low back pain. Arthritis and rheumatism. 2013 Oct:65(10):2660. doi: 10.1002/art.38069. Epub
[PubMed PMID: 23818092]
[84]
Lumezanu E, Konatalapalli R, Weinstein A. Axial (spinal) gout. Current rheumatology reports. 2012 Apr:14(2):161-4. doi: 10.1007/s11926-012-0236-8. Epub
[PubMed PMID: 22318623]
[85]
Yü TF. Secondary gout associated with myeloproliferative diseases. Arthritis and rheumatism. 1965 Oct:8(5):765-71
[PubMed PMID: 5216775]
[86]
Lin HY, Rocher LL, McQuillan MA, Schmaltz S, Palella TD, Fox IH. Cyclosporine-induced hyperuricemia and gout. The New England journal of medicine. 1989 Aug 3:321(5):287-92
[PubMed PMID: 2664517]
[87]
Choi HK, Niu J, Neogi T, Chen CA, Chaisson C, Hunter D, Zhang Y. Nocturnal risk of gout attacks. Arthritis & rheumatology (Hoboken, N.J.). 2015 Feb:67(2):555-62. doi: 10.1002/art.38917. Epub
[PubMed PMID: 25504842]
[88]
Rakieh C, Conaghan PG. Diagnosis and treatment of gout in primary care. The Practitioner. 2011 Dec:255(1746):17-20, 2-3
[PubMed PMID: 22272526]
[89]
Shah D, Mohan G, Flueckiger P, Corrigan F, Conn D. Polyarticular Gout Flare Masquerading as Sepsis. The American journal of medicine. 2015 Jul:128(7):e11-2. doi: 10.1016/j.amjmed.2014.12.025. Epub 2015 Jan 20
[PubMed PMID: 25614957]
[90]
Pascual E, Batlle-Gualda E, Martínez A, Rosas J, Vela P. Synovial fluid analysis for diagnosis of intercritical gout. Annals of internal medicine. 1999 Nov 16:131(10):756-9
[PubMed PMID: 10577299]
[91]
Coakley G, Mathews C, Field M, Jones A, Kingsley G, Walker D, Phillips M, Bradish C, McLachlan A, Mohammed R, Weston V, British Society for Rheumatology Standards, Guidelines and Audit Working Group. BSR & BHPR, BOA, RCGP and BSAC guidelines for management of the hot swollen joint in adults. Rheumatology (Oxford, England). 2006 Aug:45(8):1039-41
[PubMed PMID: 16829534]
[92]
Yu KH, Luo SF, Liou LB, Wu YJ, Tsai WP, Chen JY, Ho HH. Concomitant septic and gouty arthritis--an analysis of 30 cases. Rheumatology (Oxford, England). 2003 Sep:42(9):1062-6
[PubMed PMID: 12730521]
Level 3 (low-level) evidence
[93]
Sivera F, Andrés M, Quilis N. Gout: Diagnosis and treatment. Medicina clinica. 2017 Mar 22:148(6):271-276. doi: 10.1016/j.medcli.2016.10.019. Epub 2016 Dec 5
[PubMed PMID: 27931865]
[94]
Huppertz A, Hermann KG, Diekhoff T, Wagner M, Hamm B, Schmidt WA. Systemic staging for urate crystal deposits with dual-energy CT and ultrasound in patients with suspected gout. Rheumatology international. 2014 Jun:34(6):763-71. doi: 10.1007/s00296-014-2979-1. Epub 2014 Mar 12
[PubMed PMID: 24619560]
[95]
Ogdie A, Taylor WJ, Weatherall M, Fransen J, Jansen TL, Neogi T, Schumacher HR, Dalbeth N. Imaging modalities for the classification of gout: systematic literature review and meta-analysis. Annals of the rheumatic diseases. 2015 Oct:74(10):1868-74. doi: 10.1136/annrheumdis-2014-205431. Epub 2014 Jun 10
[PubMed PMID: 24915980]
Level 1 (high-level) evidence
[96]
Garner HW, Wessell DE. Current status of ultrasound and dual-energy computed tomography in the evaluation of gout. Rheumatology international. 2018 Aug:38(8):1339-1344. doi: 10.1007/s00296-018-4033-1. Epub 2018 May 2
[PubMed PMID: 29721694]
[97]
Li S, Xu G, Liang J, Wan L, Cao H, Lin J. The Role of Advanced Imaging in Gout Management. Frontiers in immunology. 2021:12():811323. doi: 10.3389/fimmu.2021.811323. Epub 2022 Jan 14
[PubMed PMID: 35095904]
[98]
Lee YH, Song GG. Diagnostic accuracy of ultrasound in patients with gout: A meta-analysis. Seminars in arthritis and rheumatism. 2018 Apr:47(5):703-709. doi: 10.1016/j.semarthrit.2017.09.012. Epub 2017 Sep 28
[PubMed PMID: 29054295]
Level 1 (high-level) evidence
[99]
Richette P, Doherty M, Pascual E, Barskova V, Becce F, Castañeda-Sanabria J, Coyfish M, Guillo S, Jansen TL, Janssens H, Lioté F, Mallen C, Nuki G, Perez-Ruiz F, Pimentao J, Punzi L, Pywell T, So A, Tausche AK, Uhlig T, Zavada J, Zhang W, Tubach F, Bardin T. 2016 updated EULAR evidence-based recommendations for the management of gout. Annals of the rheumatic diseases. 2017 Jan:76(1):29-42. doi: 10.1136/annrheumdis-2016-209707. Epub 2016 Jul 25
[PubMed PMID: 27457514]
[100]
FitzGerald JD, Dalbeth N, Mikuls T, Brignardello-Petersen R, Guyatt G, Abeles AM, Gelber AC, Harrold LR, Khanna D, King C, Levy G, Libbey C, Mount D, Pillinger MH, Rosenthal A, Singh JA, Sims JE, Smith BJ, Wenger NS, Bae SS, Danve A, Khanna PP, Kim SC, Lenert A, Poon S, Qasim A, Sehra ST, Sharma TSK, Toprover M, Turgunbaev M, Zeng L, Zhang MA, Turner AS, Neogi T. 2020 American College of Rheumatology Guideline for the Management of Gout. Arthritis care & research. 2020 Jun:72(6):744-760. doi: 10.1002/acr.24180. Epub 2020 May 11
[PubMed PMID: 32391934]
[101]
Schlesinger N, Detry MA, Holland BK, Baker DG, Beutler AM, Rull M, Hoffman BI, Schumacher HR Jr. Local ice therapy during bouts of acute gouty arthritis. The Journal of rheumatology. 2002 Feb:29(2):331-4
[PubMed PMID: 11838852]
[102]
Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, Pillinger MH, Merill J, Lee S, Prakash S, Kaldas M, Gogia M, Perez-Ruiz F, Taylor W, Lioté F, Choi H, Singh JA, Dalbeth N, Kaplan S, Niyyar V, Jones D, Yarows SA, Roessler B, Kerr G, King C, Levy G, Furst DE, Edwards NL, Mandell B, Schumacher HR, Robbins M, Wenger N, Terkeltaub R, American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis care & research. 2012 Oct:64(10):1431-46. doi: 10.1002/acr.21772. Epub
[PubMed PMID: 23024028]
Level 1 (high-level) evidence
[103]
Zhang W, Doherty M, Bardin T, Pascual E, Barskova V, Conaghan P, Gerster J, Jacobs J, Leeb B, Lioté F, McCarthy G, Netter P, Nuki G, Perez-Ruiz F, Pignone A, Pimentão J, Punzi L, Roddy E, Uhlig T, Zimmermann-Gòrska I, EULAR Standing Committee for International Clinical Studies Including Therapeutics. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Annals of the rheumatic diseases. 2006 Oct:65(10):1312-24
[PubMed PMID: 16707532]
[104]
Zhang W, Doherty M, Pascual E, Bardin T, Barskova V, Conaghan P, Gerster J, Jacobs J, Leeb B, Lioté F, McCarthy G, Netter P, Nuki G, Perez-Ruiz F, Pignone A, Pimentão J, Punzi L, Roddy E, Uhlig T, Zimmermann-Gòrska I, EULAR Standing Committee for International Clinical Studies Including Therapeutics. EULAR evidence based recommendations for gout. Part I: Diagnosis. Report of a task force of the Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Annals of the rheumatic diseases. 2006 Oct:65(10):1301-11
[PubMed PMID: 16707533]
[105]
YU TF, GUTMAN AB. Study of the paradoxical effects of salicylate in low, intermediate and high dosage on the renal mechanisms for excretion of urate in man. The Journal of clinical investigation. 1959 Aug:38(8):1298-315
[PubMed PMID: 13673086]
[106]
YUE TF, DAYTON PG, GUTMAN AB. MUTUAL SUPPRESSION OF THE URICOSURIC EFFECTS OF SULFINPYRAZONE AND SALICYLATE: A STUDY IN INTERACTIONS BETWEEN DRUGS. The Journal of clinical investigation. 1963 Aug:42(8):1330-9
[PubMed PMID: 14060403]
[107]
Caspi D, Lubart E, Graff E, Habot B, Yaron M, Segal R. The effect of mini-dose aspirin on renal function and uric acid handling in elderly patients. Arthritis and rheumatism. 2000 Jan:43(1):103-8
[PubMed PMID: 10643705]
[108]
Segal R, Lubart E, Leibovitz A, Iaina A, Caspi D. Renal effects of low dose aspirin in elderly patients. The Israel Medical Association journal : IMAJ. 2006 Oct:8(10):679-82
[PubMed PMID: 17125112]
[109]
Zhang YK, Yang H, Zhang JY, Song LJ, Fan YC. Comparison of intramuscular compound betamethasone and oral diclofenac sodium in the treatment of acute attacks of gout. International journal of clinical practice. 2014 May:68(5):633-8. doi: 10.1111/ijcp.12359. Epub 2014 Jan 29
[PubMed PMID: 24472084]
[110]
Alloway JA, Moriarty MJ, Hoogland YT, Nashel DJ. Comparison of triamcinolone acetonide with indomethacin in the treatment of acute gouty arthritis. The Journal of rheumatology. 1993 Jan:20(1):111-3
[PubMed PMID: 8441139]
[111]
Rainer TH, Cheng CH, Janssens HJ, Man CY, Tam LS, Choi YF, Yau WH, Lee KH, Graham CA. Oral Prednisolone in the Treatment of Acute Gout: A Pragmatic, Multicenter, Double-Blind, Randomized Trial. Annals of internal medicine. 2016 Apr 5:164(7):464-71. doi: 10.7326/M14-2070. Epub 2016 Feb 23
[PubMed PMID: 26903390]
Level 1 (high-level) evidence
[112]
Yu J, Lu H, Zhou J, Xie Z, Wen C, Xu Z. Oral prednisolone versus non-steroidal anti-inflammatory drugs in the treatment of acute gout: a meta-analysis of randomized controlled trials. Inflammopharmacology. 2018 Jun:26(3):717-723. doi: 10.1007/s10787-018-0442-8. Epub 2018 Jan 22
[PubMed PMID: 29357007]
Level 1 (high-level) evidence
[113]
Zeng L, Qasim A, Neogi T, Fitzgerald JD, Dalbeth N, Mikuls TR, Guyatt GH, Brignardello-Petersen R. Efficacy and Safety of Pharmacologic Interventions in Patients Experiencing a Gout Flare: A Systematic Review and Network Meta-Analysis. Arthritis care & research. 2021 May:73(5):755-764. doi: 10.1002/acr.24402. Epub
[PubMed PMID: 32741131]
Level 1 (high-level) evidence
[114]
Imazio M, Nidorf M. Colchicine and the heart. European heart journal. 2021 Jul 21:42(28):2745-2760. doi: 10.1093/eurheartj/ehab221. Epub
[PubMed PMID: 33961006]
[115]
Terkeltaub RA, Furst DE, Bennett K, Kook KA, Crockett RS, Davis MW. High versus low dosing of oral colchicine for early acute gout flare: Twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis and rheumatism. 2010 Apr:62(4):1060-8. doi: 10.1002/art.27327. Epub
[PubMed PMID: 20131255]
Level 1 (high-level) evidence
[116]
. Colchicine and other drugs for gout. The Medical letter on drugs and therapeutics. 2009 Nov 30:51(1326):93-4
[PubMed PMID: 20224523]
Level 3 (low-level) evidence
[117]
Todd BA, Billups SJ, Delate T, Canty KE, Kauffman AB, Rawlings JE, Wagner TM. Assessment of the association between colchicine therapy and serious adverse events. Pharmacotherapy. 2012 Nov:32(11):974-80. doi: 10.1002/phar.1125. Epub 2012 Sep 27
[PubMed PMID: 23019065]
[118]
Terkeltaub RA. Colchicine update: 2008. Seminars in arthritis and rheumatism. 2009 Jun:38(6):411-9. doi: 10.1016/j.semarthrit.2008.08.006. Epub 2008 Oct 29
[PubMed PMID: 18973929]
[119]
Kurup R, Galougahi KK, Figtree G, Misra A, Patel S. The Role of Colchicine in Atherosclerotic Cardiovascular Disease. Heart, lung & circulation. 2021 Jun:30(6):795-806. doi: 10.1016/j.hlc.2020.11.010. Epub 2021 Jan 16
[PubMed PMID: 33461916]
[120]
Deftereos SG, Beerkens FJ, Shah B, Giannopoulos G, Vrachatis DA, Giotaki SG, Siasos G, Nicolas J, Arnott C, Patel S, Parsons M, Tardif JC, Kovacic JC, Dangas GD. Colchicine in Cardiovascular Disease: In-Depth Review. Circulation. 2022 Jan 4:145(1):61-78. doi: 10.1161/CIRCULATIONAHA.121.056171. Epub 2021 Dec 29
[PubMed PMID: 34965168]
[121]
Solomon DH, Liu CC, Kuo IH, Zak A, Kim SC. Effects of colchicine on risk of cardiovascular events and mortality among patients with gout: a cohort study using electronic medical records linked with Medicare claims. Annals of the rheumatic diseases. 2016 Sep:75(9):1674-9. doi: 10.1136/annrheumdis-2015-207984. Epub 2015 Nov 18
[PubMed PMID: 26582823]
[122]
Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, Berry C, López-Sendón J, Ostadal P, Koenig W, Angoulvant D, Grégoire JC, Lavoie MA, Dubé MP, Rhainds D, Provencher M, Blondeau L, Orfanos A, L'Allier PL, Guertin MC, Roubille F. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. The New England journal of medicine. 2019 Dec 26:381(26):2497-2505. doi: 10.1056/NEJMoa1912388. Epub 2019 Nov 16
[PubMed PMID: 31733140]
[123]
Paulus HE, Schlosstein LH, Godfrey RG, Klinenberg JR, Bluestone R. Prophylactic colchicine therapy of intercritical gout. A placebo-controlled study of probenecid-treated patients. Arthritis and rheumatism. 1974 Sep-Oct:17(5):609-14
[PubMed PMID: 4606955]
[124]
Borstad GC, Bryant LR, Abel MP, Scroggie DA, Harris MD, Alloway JA. Colchicine for prophylaxis of acute flares when initiating allopurinol for chronic gouty arthritis. The Journal of rheumatology. 2004 Dec:31(12):2429-32
[PubMed PMID: 15570646]
[125]
Latourte A, Bardin T, Richette P. Prophylaxis for acute gout flares after initiation of urate-lowering therapy. Rheumatology (Oxford, England). 2014 Nov:53(11):1920-6. doi: 10.1093/rheumatology/keu157. Epub 2014 Apr 23
[PubMed PMID: 24758886]
[126]
Liew JW, Gardner GC. Use of Anakinra in Hospitalized Patients with Crystal-associated Arthritis. The Journal of rheumatology. 2019 Oct:46(10):1345-1349. doi: 10.3899/jrheum.181018. Epub 2019 Jan 15
[PubMed PMID: 30647192]
[127]
Saag KG, Khanna PP, Keenan RT, Ohlman S, Osterling Koskinen L, Sparve E, Åkerblad AC, Wikén M, So A, Pillinger MH, Terkeltaub R. A Randomized, Phase II Study Evaluating the Efficacy and Safety of Anakinra in the Treatment of Gout Flares. Arthritis & rheumatology (Hoboken, N.J.). 2021 Aug:73(8):1533-1542. doi: 10.1002/art.41699. Epub 2021 Jul 7
[PubMed PMID: 33605029]
Level 1 (high-level) evidence
[128]
So A, De Meulemeester M, Pikhlak A, Yücel AE, Richard D, Murphy V, Arulmani U, Sallstig P, Schlesinger N. Canakinumab for the treatment of acute flares in difficult-to-treat gouty arthritis: Results of a multicenter, phase II, dose-ranging study. Arthritis and rheumatism. 2010 Oct:62(10):3064-76. doi: 10.1002/art.27600. Epub
[PubMed PMID: 20533546]
[129]
Schlesinger N, Alten RE, Bardin T, Schumacher HR, Bloch M, Gimona A, Krammer G, Murphy V, Richard D, So AK. Canakinumab for acute gouty arthritis in patients with limited treatment options: results from two randomised, multicentre, active-controlled, double-blind trials and their initial extensions. Annals of the rheumatic diseases. 2012 Nov:71(11):1839-48. doi: 10.1136/annrheumdis-2011-200908. Epub 2012 May 14
[PubMed PMID: 22586173]
Level 1 (high-level) evidence
[130]
Kiltz U, Smolen J, Bardin T, Cohen Solal A, Dalbeth N, Doherty M, Engel B, Flader C, Kay J, Matsuoka M, Perez-Ruiz F, da Rocha Castelar-Pinheiro G, Saag K, So A, Vazquez Mellado J, Weisman M, Westhoff TH, Yamanaka H, Braun J. Treat-to-target (T2T) recommendations for gout. Annals of the rheumatic diseases. 2017 Apr:76(4):632-638. doi: 10.1136/annrheumdis-2016-209467. Epub 2016 Sep 22
[PubMed PMID: 27658678]
[131]
Taylor TH, Mecchella JN, Larson RJ, Kerin KD, Mackenzie TA. Initiation of allopurinol at first medical contact for acute attacks of gout: a randomized clinical trial. The American journal of medicine. 2012 Nov:125(11):1126-1134.e7. doi: 10.1016/j.amjmed.2012.05.025. Epub
[PubMed PMID: 23098865]
Level 1 (high-level) evidence
[132]
Hill EM, Sky K, Sit M, Collamer A, Higgs J. Does starting allopurinol prolong acute treated gout? A randomized clinical trial. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. 2015 Apr:21(3):120-5. doi: 10.1097/RHU.0000000000000235. Epub
[PubMed PMID: 25807090]
Level 1 (high-level) evidence
[133]
Jia E, Zhang Y, Ma W, Li B, Geng H, Zhong L, Yao X, Xie J, Xiao Y, Jiang Y, Qiu X, Xiao M, Cui X, Wei J, Zhang J. Initiation of febuxostat for acute gout flare does not prolong the current episode: a randomized clinical trial. Rheumatology (Oxford, England). 2021 Sep 1:60(9):4199-4204. doi: 10.1093/rheumatology/keaa908. Epub
[PubMed PMID: 33404656]
Level 1 (high-level) evidence
[134]
Hung SI,Chung WH,Liou LB,Chu CC,Lin M,Huang HP,Lin YL,Lan JL,Yang LC,Hong HS,Chen MJ,Lai PC,Wu MS,Chu CY,Wang KH,Chen CH,Fann CS,Wu JY,Chen YT, HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proceedings of the National Academy of Sciences of the United States of America. 2005 Mar 15;
[PubMed PMID: 15743917]
[135]
Stamp LK, Taylor WJ, Jones PB, Dockerty JL, Drake J, Frampton C, Dalbeth N. Starting dose is a risk factor for allopurinol hypersensitivity syndrome: a proposed safe starting dose of allopurinol. Arthritis and rheumatism. 2012 Aug:64(8):2529-36. doi: 10.1002/art.34488. Epub
[PubMed PMID: 22488501]
[136]
Stamp LK, O'Donnell JL, Zhang M, James J, Frampton C, Barclay ML, Chapman PT. Using allopurinol above the dose based on creatinine clearance is effective and safe in patients with chronic gout, including those with renal impairment. Arthritis and rheumatism. 2011 Feb:63(2):412-21. doi: 10.1002/art.30119. Epub
[PubMed PMID: 21279998]
[137]
Sarawate CA, Patel PA, Schumacher HR, Yang W, Brewer KK, Bakst AW. Serum urate levels and gout flares: analysis from managed care data. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. 2006 Apr:12(2):61-5
[PubMed PMID: 16601538]
[138]
Ragab AH, Gilkerson E, Myers M. The effect of 6-mercaptopurine and allopurinol on granulopoiesis. Cancer research. 1974 Sep:34(9):2246-9
[PubMed PMID: 4843532]
[139]
Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, Streit J, Joseph-Ridge N. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. The New England journal of medicine. 2005 Dec 8:353(23):2450-61
[PubMed PMID: 16339094]
[140]
Becker MA, Schumacher HR, MacDonald PA, Lloyd E, Lademacher C. Clinical efficacy and safety of successful longterm urate lowering with febuxostat or allopurinol in subjects with gout. The Journal of rheumatology. 2009 Jun:36(6):1273-82. doi: 10.3899/jrheum.080814. Epub 2009 Mar 13
[PubMed PMID: 19286847]
[141]
White WB, Saag KG, Becker MA, Borer JS, Gorelick PB, Whelton A, Hunt B, Castillo M, Gunawardhana L, CARES Investigators. Cardiovascular Safety of Febuxostat or Allopurinol in Patients with Gout. The New England journal of medicine. 2018 Mar 29:378(13):1200-1210. doi: 10.1056/NEJMoa1710895. Epub 2018 Mar 12
[PubMed PMID: 29527974]
[142]
Su CY, Shen LJ, Hsieh SC, Lin LY, Lin FJ. Comparing Cardiovascular Safety of Febuxostat and Allopurinol in the Real World: A Population-Based Cohort Study. Mayo Clinic proceedings. 2019 Jul:94(7):1147-1157. doi: 10.1016/j.mayocp.2019.03.001. Epub
[PubMed PMID: 31272565]
[143]
Weisshaar S, Litschauer B, Reichardt B, Gruber F, Leitner S, Sibinovic S, Kossmeier M, Wolzt M. Cardiovascular risk and mortality in patients with hyperuricemia treated with febuxostat or allopurinol: a retrospective nation-wide cohort study in Austria 2014-2017. Rheumatology international. 2022 Sep:42(9):1597-1603. doi: 10.1007/s00296-022-05139-8. Epub 2022 May 19
[PubMed PMID: 35589988]
Level 2 (mid-level) evidence
[144]
Mackenzie IS, Ford I, Nuki G, Hallas J, Hawkey CJ, Webster J, Ralston SH, Walters M, Robertson M, De Caterina R, Findlay E, Perez-Ruiz F, McMurray JJV, MacDonald TM, FAST Study Group. Long-term cardiovascular safety of febuxostat compared with allopurinol in patients with gout (FAST): a multicentre, prospective, randomised, open-label, non-inferiority trial. Lancet (London, England). 2020 Nov 28:396(10264):1745-1757. doi: 10.1016/S0140-6736(20)32234-0. Epub 2020 Nov 9
[PubMed PMID: 33181081]
Level 1 (high-level) evidence
[145]
Kang EH, Choi HK, Shin A, Lee YJ, Lee EB, Song YW, Kim SC. Comparative cardiovascular risk of allopurinol versus febuxostat in patients with gout: a nation-wide cohort study. Rheumatology (Oxford, England). 2019 Dec 1:58(12):2122-2129. doi: 10.1093/rheumatology/kez189. Epub
[PubMed PMID: 31098635]
Level 2 (mid-level) evidence
[146]
Jeong H, Choi E, Suh A, Yoo M, Kim B. `Risk of cardiovascular disease associated with febuxostat versus allopurinol use in patients with gout: a retrospective cohort study in Korea. Rheumatology international. 2023 Feb:43(2):265-281. doi: 10.1007/s00296-022-05222-0. Epub 2022 Nov 8
[PubMed PMID: 36346443]
Level 2 (mid-level) evidence
[147]
Gao L, Wang B, Pan Y, Lu Y, Cheng R. Cardiovascular safety of febuxostat compared to allopurinol for the treatment of gout: A systematic and meta-analysis. Clinical cardiology. 2021 Jul:44(7):907-916. doi: 10.1002/clc.23643. Epub 2021 May 20
[PubMed PMID: 34013998]
Level 1 (high-level) evidence
[148]
Ghang BZ, Lee JS, Choi J, Kim J, Yoo B. Increased risk of cardiovascular events and death in the initial phase after discontinuation of febuxostat or allopurinol: another story of the CARES trial. RMD open. 2022 Jun:8(2):. doi: 10.1136/rmdopen-2021-001944. Epub
[PubMed PMID: 35732345]
[149]
O'Dell JR, Brophy MT, Pillinger MH, Neogi T, Palevsky PM, Wu H, Davis-Karim A, Newcomb JA, Ferguson R, Pittman D, Cannon GW, Taylor T, Terkeltaub R, Cannella AC, England BR, Helget LN, Mikuls TR. Comparative Effectiveness of Allopurinol and Febuxostat in Gout Management. NEJM evidence. 2022 Mar:1(3):. doi: 10.1056/evidoa2100028. Epub 2022 Feb 3
[PubMed PMID: 35434725]
Level 2 (mid-level) evidence
[150]
Gupta MK, Singh JA. Cardiovascular Disease in Gout and the Protective Effect of Treatments Including Urate-Lowering Therapy. Drugs. 2019 Apr:79(5):531-541. doi: 10.1007/s40265-019-01081-5. Epub
[PubMed PMID: 30868398]
[151]
Dubreuil M, Zhu Y, Zhang Y, Seeger JD, Lu N, Rho YH, Choi HK. Allopurinol initiation and all-cause mortality in the general population. Annals of the rheumatic diseases. 2015 Jul:74(7):1368-72. doi: 10.1136/annrheumdis-2014-205269. Epub 2014 Mar 24
[PubMed PMID: 24665118]
[152]
Luk AJ,Levin GP,Moore EE,Zhou XH,Kestenbaum BR,Choi HK, Allopurinol and mortality in hyperuricaemic patients. Rheumatology (Oxford, England). 2009 Jul
[PubMed PMID: 19447769]
[153]
Chen JH, Lan JL, Cheng CF, Liang WM, Lin HY, Tsay GJ, Yeh WT, Pan WH. Effect of Urate-lowering Therapy on the Risk of Cardiovascular Disease and All-cause Mortality in Patients with Gout: A Case-matched Cohort Study. The Journal of rheumatology. 2015 Sep:42(9):1694-701. doi: 10.3899/jrheum.141542. Epub 2015 Jun 15
[PubMed PMID: 26077411]
Level 3 (low-level) evidence
[154]
Singh JA, Yu S. Allopurinol and the risk of atrial fibrillation in the elderly: a study using Medicare data. Annals of the rheumatic diseases. 2017 Jan:76(1):72-78. doi: 10.1136/annrheumdis-2015-209008. Epub 2016 May 10
[PubMed PMID: 27165177]
[155]
Vargas-Santos AB, Peloquin CE, Zhang Y, Neogi T. Association of Chronic Kidney Disease With Allopurinol Use in Gout Treatment. JAMA internal medicine. 2018 Nov 1:178(11):1526-1533. doi: 10.1001/jamainternmed.2018.4463. Epub
[PubMed PMID: 30304329]
[156]
Singh JA, Yu S. Are allopurinol dose and duration of use nephroprotective in the elderly? A Medicare claims study of allopurinol use and incident renal failure. Annals of the rheumatic diseases. 2017 Jan:76(1):133-139. doi: 10.1136/annrheumdis-2015-209046. Epub 2016 Jun 13
[PubMed PMID: 27296322]
[157]
Levy GD, Rashid N, Niu F, Cheetham TC. Effect of urate-lowering therapies on renal disease progression in patients with hyperuricemia. The Journal of rheumatology. 2014 May:41(5):955-62. doi: 10.3899/jrheum.131159. Epub 2014 Apr 1
[PubMed PMID: 24692523]
[158]
Kim Y, Shin S, Kim K, Choi S, Lee K. Effect of Urate Lowering Therapy on Renal Disease Progression in Hyperuricemic Patients with Chronic Kidney Disease. The Journal of rheumatology. 2015 Nov:42(11):2143-8. doi: 10.3899/jrheum.150067. Epub 2015 Oct 1
[PubMed PMID: 26428209]
[159]
Singh JA, Cleveland JD. Comparative effectiveness of allopurinol versus febuxostat for preventing incident renal disease in older adults: an analysis of Medicare claims data. Annals of the rheumatic diseases. 2017 Oct:76(10):1669-1678. doi: 10.1136/annrheumdis-2017-211210. Epub 2017 Jun 5
[PubMed PMID: 28584186]
Level 2 (mid-level) evidence
[160]
Weinberger A, Schindel B, Liberman UA, Pinkhas J, Sperling O. Calciuric effect of probenecid in gouty patients. Israel journal of medical sciences. 1983 Apr:19(4):377-9
[PubMed PMID: 6853140]
[161]
Schlesinger N, Yasothan U, Kirkpatrick P. Pegloticase. Nature reviews. Drug discovery. 2011 Jan:10(1):17-8. doi: 10.1038/nrd3349. Epub
[PubMed PMID: 21193861]
Level 3 (low-level) evidence
[162]
Sundy JS, Baraf HS, Yood RA, Edwards NL, Gutierrez-Urena SR, Treadwell EL, Vázquez-Mellado J, White WB, Lipsky PE, Horowitz Z, Huang W, Maroli AN, Waltrip RW 2nd, Hamburger SA, Becker MA. Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: two randomized controlled trials. JAMA. 2011 Aug 17:306(7):711-20. doi: 10.1001/jama.2011.1169. Epub
[PubMed PMID: 21846852]
Level 1 (high-level) evidence
[163]
Becker MA, Baraf HS, Yood RA, Dillon A, Vázquez-Mellado J, Ottery FD, Khanna D, Sundy JS. Long-term safety of pegloticase in chronic gout refractory to conventional treatment. Annals of the rheumatic diseases. 2013 Sep 1:72(9):1469-74. doi: 10.1136/annrheumdis-2012-201795. Epub 2012 Nov 10
[PubMed PMID: 23144450]
Level 3 (low-level) evidence
[164]
Keenan RT, Yeo AE, Lipsky PE. Pegloticase causes prolonged improvement in multiple disease parameters in patients with chronic refractory gout who maintain low serum urate levels. Clinical and experimental rheumatology. 2022 May:40(5):1006-1010. doi: 10.55563/clinexprheumatol/3m095f. Epub 2022 Feb 25
[PubMed PMID: 35238750]
Level 3 (low-level) evidence
[165]
Leung N, Yip K, Pillinger MH, Toprover M. Lowering and Raising Serum Urate Levels: Off-Label Effects of Commonly Used Medications. Mayo Clinic proceedings. 2022 Jul:97(7):1345-1362. doi: 10.1016/j.mayocp.2022.02.027. Epub
[PubMed PMID: 35787862]
[166]
Zhao Y, Xu L, Tian D, Xia P, Zheng H, Wang L, Chen L. Effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on serum uric acid level: A meta-analysis of randomized controlled trials. Diabetes, obesity & metabolism. 2018 Feb:20(2):458-462. doi: 10.1111/dom.13101. Epub 2017 Sep 27
[PubMed PMID: 28846182]
Level 1 (high-level) evidence
[167]
Doehner W, Anker SD, Butler J, Zannad F, Filippatos G, Ferreira JP, Salsali A, Kaempfer C, Brueckmann M, Pocock SJ, Januzzi JL, Packer M. Uric acid and sodium-glucose cotransporter-2 inhibition with empagliflozin in heart failure with reduced ejection fraction: the EMPEROR-reduced trial. European heart journal. 2022 Sep 21:43(36):3435-3446. doi: 10.1093/eurheartj/ehac320. Epub
[PubMed PMID: 35788657]
[168]
Fralick M, Chen SK, Patorno E, Kim SC. Assessing the Risk for Gout With Sodium-Glucose Cotransporter-2 Inhibitors in Patients With Type 2 Diabetes: A Population-Based Cohort Study. Annals of internal medicine. 2020 Feb 4:172(3):186-194. doi: 10.7326/M19-2610. Epub 2020 Jan 14
[PubMed PMID: 31931526]
[169]
Berman EL. Clues in the eye: ocular signs of metabolic and nutritional disorders. Geriatrics. 1995 Jul:50(7):34-6, 43-4
[PubMed PMID: 7601360]
[170]
Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Archives of internal medicine. 2005 Apr 11:165(7):742-8
[PubMed PMID: 15824292]
[171]
Roubenoff R, Klag MJ, Mead LA, Liang KY, Seidler AJ, Hochberg MC. Incidence and risk factors for gout in white men. JAMA. 1991 Dec 4:266(21):3004-7
[PubMed PMID: 1820473]
[172]
Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006 Mar 9:440(7081):237-41
[PubMed PMID: 16407889]
[173]
Singh JA. Gout and comorbidity: a nominal group study of people with gout. Arthritis research & therapy. 2017 Sep 15:19(1):204. doi: 10.1186/s13075-017-1416-8. Epub 2017 Sep 15
[PubMed PMID: 28915838]

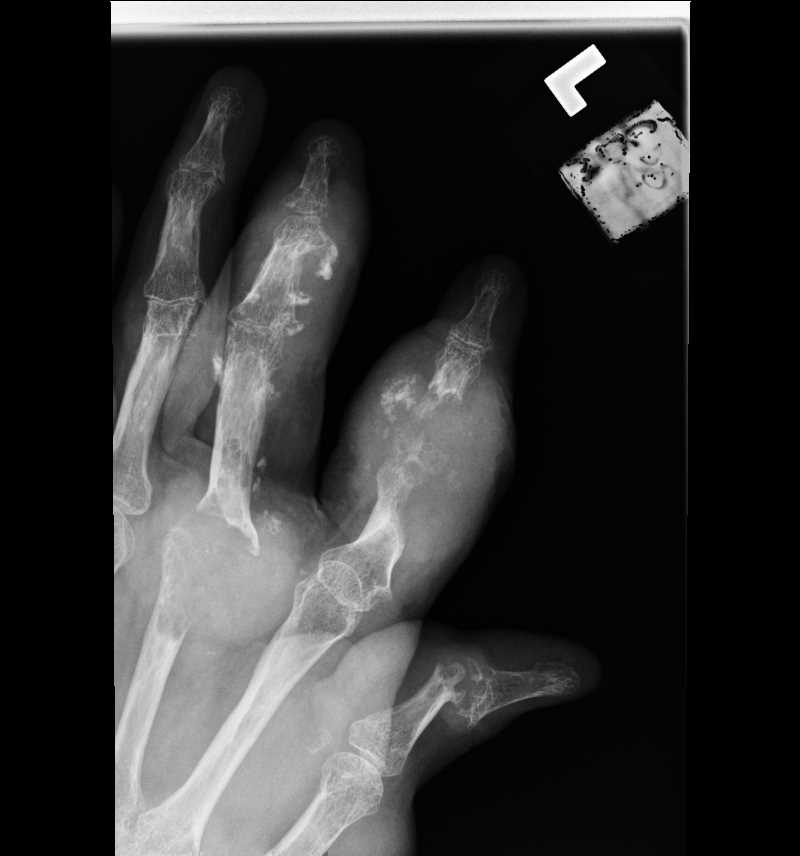
![Gout, [SATA]](/pictures/getimagecontent//6491)