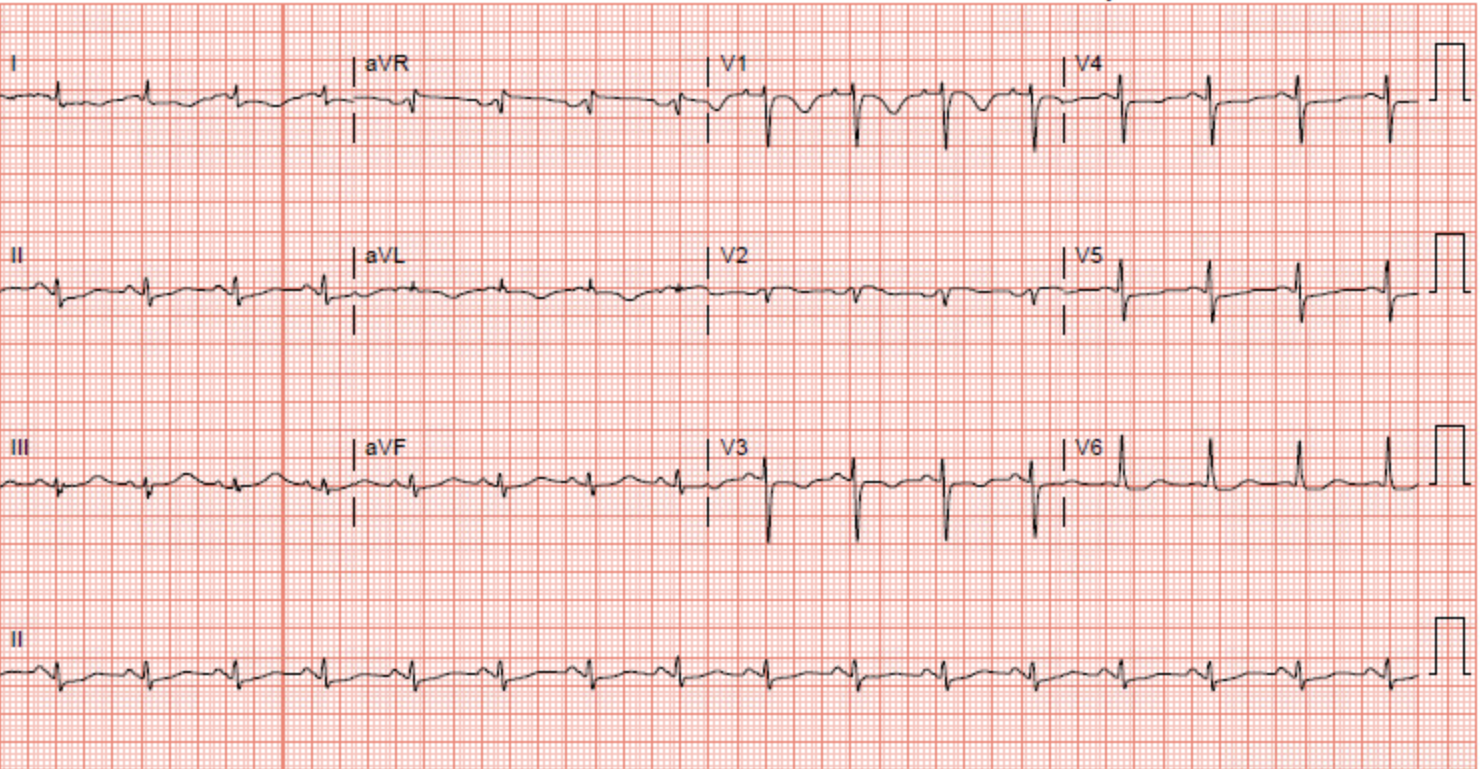
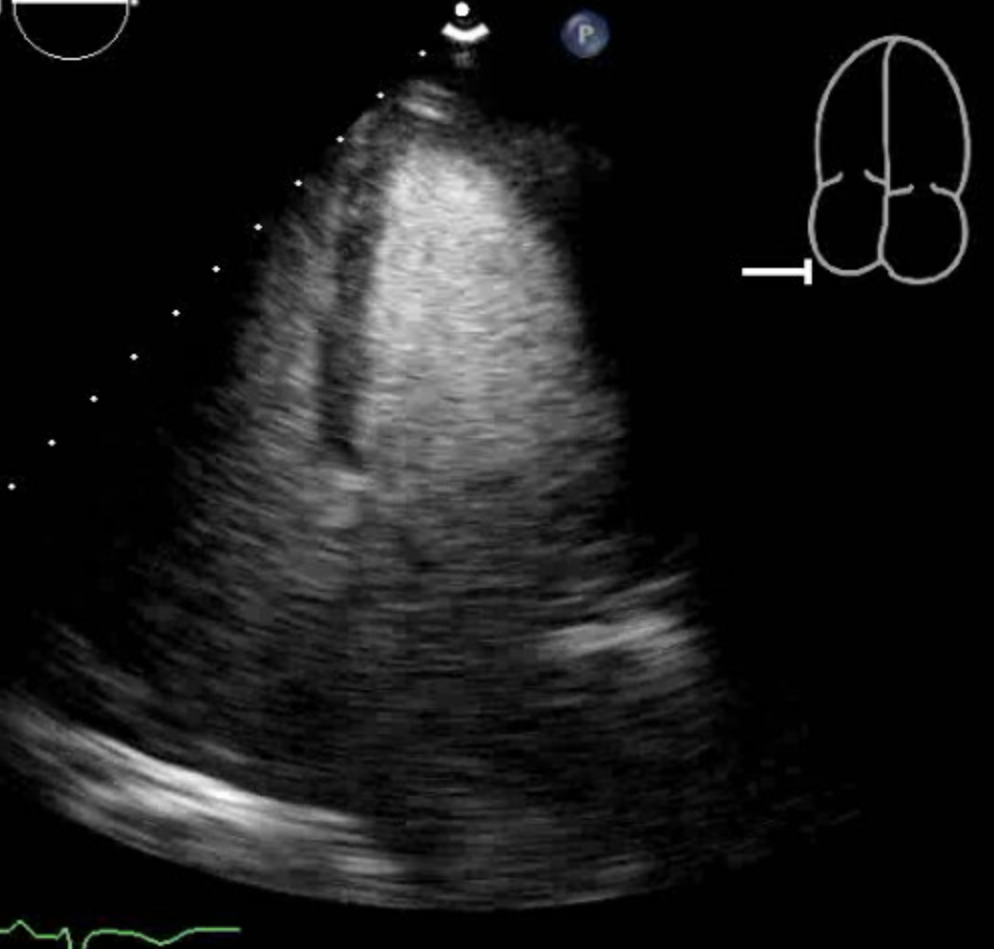
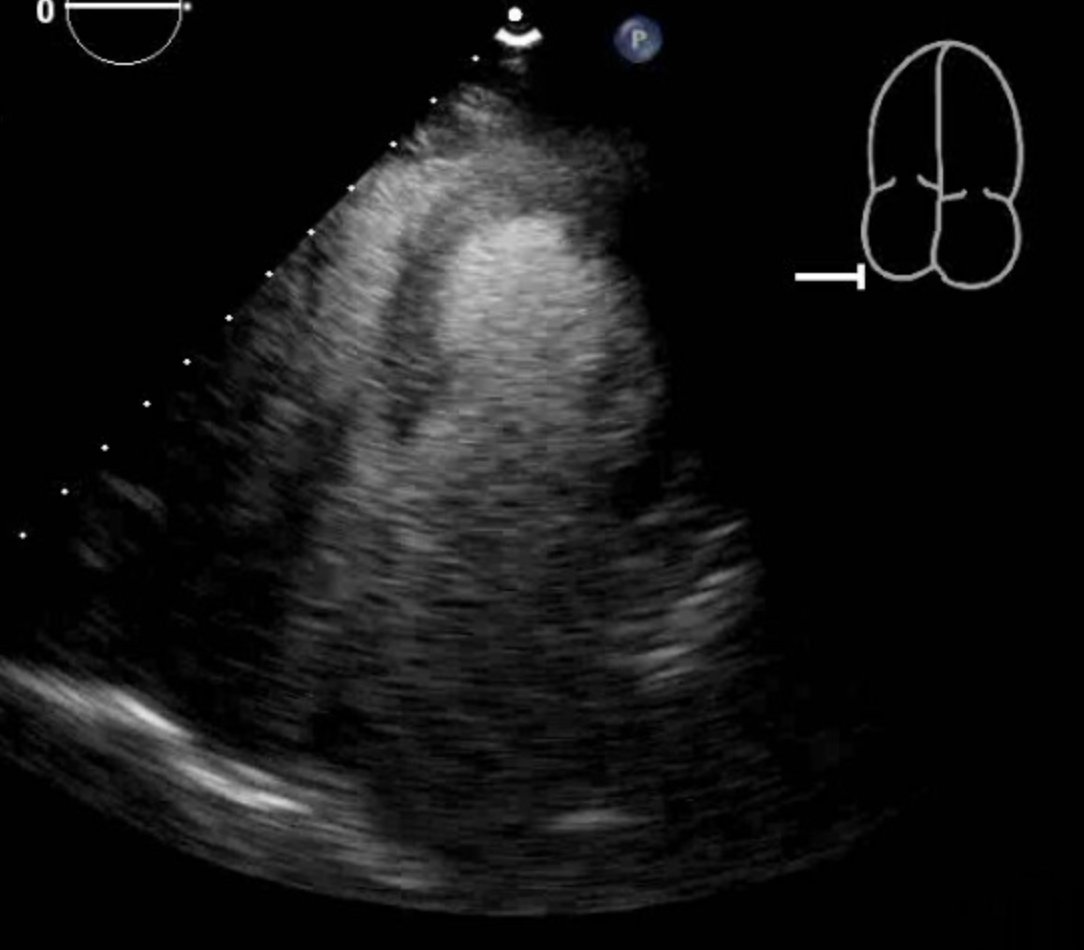
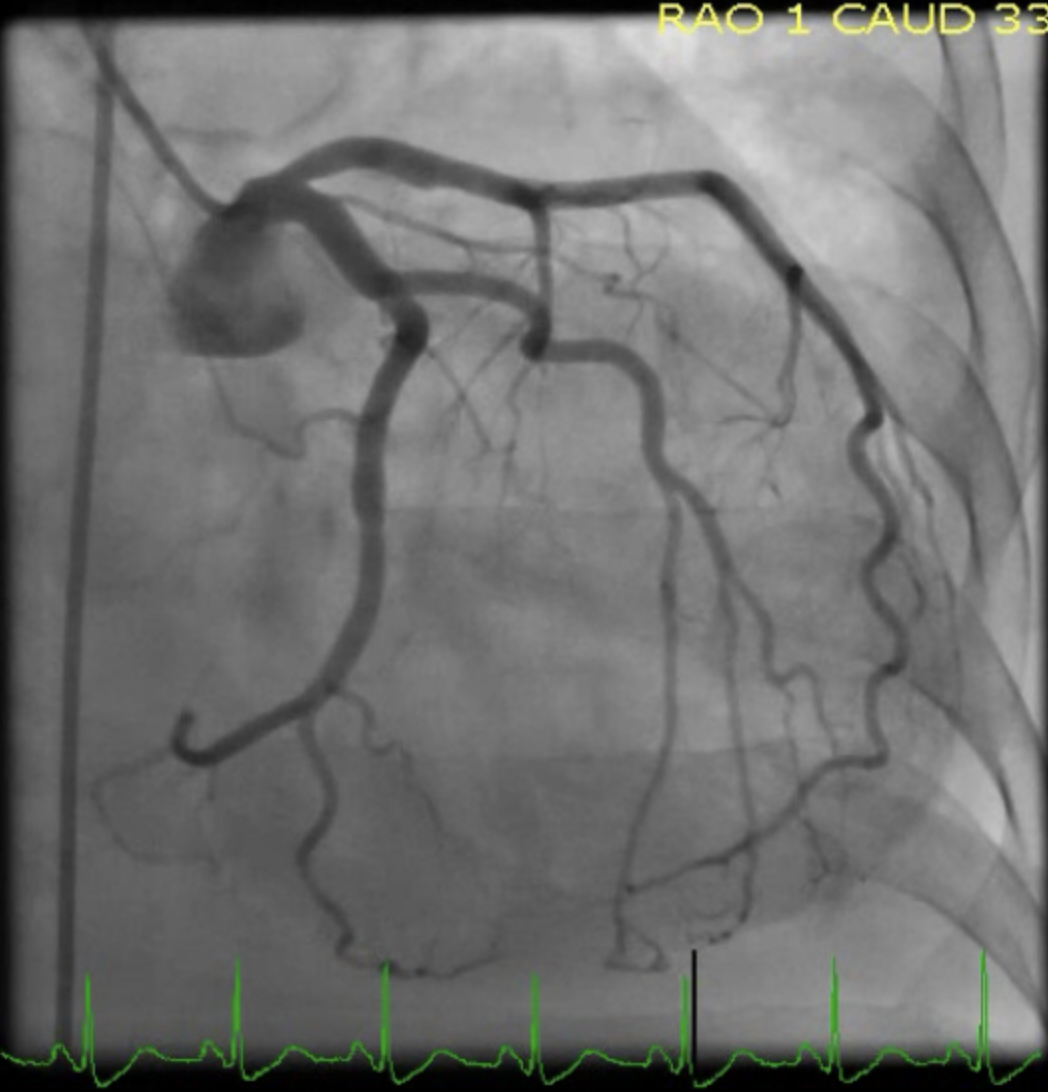
[1]
Roshanzamir S, Showkathali R. Takotsubo cardiomyopathy a short review. Current cardiology reviews. 2013 Aug:9(3):191-6
[PubMed PMID: 23642025]
[2]
Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, Cammann VL, Crea F, Galiuto L, Desmet W, Yoshida T, Manfredini R, Eitel I, Kosuge M, Nef HM, Deshmukh A, Lerman A, Bossone E, Citro R, Ueyama T, Corrado D, Kurisu S, Ruschitzka F, Winchester D, Lyon AR, Omerovic E, Bax JJ, Meimoun P, Tarantini G, Rihal C, Y-Hassan S, Migliore F, Horowitz JD, Shimokawa H, Lüscher TF, Templin C. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. European heart journal. 2018 Jun 7:39(22):2032-2046. doi: 10.1093/eurheartj/ehy076. Epub
[PubMed PMID: 29850871]
Level 3 (low-level) evidence
[3]
Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. European heart journal. 2006 Jul:27(13):1523-9
[PubMed PMID: 16720686]
Level 1 (high-level) evidence
[4]
Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, Seifert B, Hellermann J, Schwyzer M, Eisenhardt K, Jenewein J, Franke J, Katus HA, Burgdorf C, Schunkert H, Moeller C, Thiele H, Bauersachs J, Tschöpe C, Schultheiss HP, Laney CA, Rajan L, Michels G, Pfister R, Ukena C, Böhm M, Erbel R, Cuneo A, Kuck KH, Jacobshagen C, Hasenfuss G, Karakas M, Koenig W, Rottbauer W, Said SM, Braun-Dullaeus RC, Cuculi F, Banning A, Fischer TA, Vasankari T, Airaksinen KE, Fijalkowski M, Rynkiewicz A, Pawlak M, Opolski G, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Crea F, Dichtl W, Franz WM, Empen K, Felix SB, Delmas C, Lairez O, Erne P, Bax JJ, Ford I, Ruschitzka F, Prasad A, Lüscher TF. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. The New England journal of medicine. 2015 Sep 3:373(10):929-38. doi: 10.1056/NEJMoa1406761. Epub
[PubMed PMID: 26332547]
[5]
Nef HM, Möllmann H, Kostin S, Troidl C, Voss S, Weber M, Dill T, Rolf A, Brandt R, Hamm CW, Elsässer A. Tako-Tsubo cardiomyopathy: intraindividual structural analysis in the acute phase and after functional recovery. European heart journal. 2007 Oct:28(20):2456-64
[PubMed PMID: 17395683]
[6]
Karch SB, Billingham ME. Myocardial contraction bands revisited. Human pathology. 1986 Jan:17(1):9-13
[PubMed PMID: 2417934]
[7]
Fineschi V, Silver MD, Karch SB, Parolini M, Turillazzi E, Pomara C, Baroldi G. Myocardial disarray: an architectural disorganization linked with adrenergic stress? International journal of cardiology. 2005 Mar 18:99(2):277-82
[PubMed PMID: 15749187]
[8]
Kurisu S, Sato H, Kawagoe T, Ishihara M, Shimatani Y, Nishioka K, Kono Y, Umemura T, Nakamura S. Tako-tsubo-like left ventricular dysfunction with ST-segment elevation: a novel cardiac syndrome mimicking acute myocardial infarction. American heart journal. 2002 Mar:143(3):448-55
[PubMed PMID: 11868050]
[9]
Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC. Neurohumoral features of myocardial stunning due to sudden emotional stress. The New England journal of medicine. 2005 Feb 10:352(6):539-48
[PubMed PMID: 15703419]
[10]
Parkkonen O, Allonen J, Vaara S, Viitasalo M, Nieminen MS, Sinisalo J. Differences in ST-elevation and T-wave amplitudes do not reliably differentiate takotsubo cardiomyopathy from acute anterior myocardial infarction. Journal of electrocardiology. 2014 Sep-Oct:47(5):692-9. doi: 10.1016/j.jelectrocard.2014.06.006. Epub 2014 Jun 14
[PubMed PMID: 25022798]
[11]
Tsuchihashi K, Ueshima K, Uchida T, Oh-mura N, Kimura K, Owa M, Yoshiyama M, Miyazaki S, Haze K, Ogawa H, Honda T, Hase M, Kai R, Morii I, Angina Pectoris-Myocardial Infarction Investigations in Japan. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina Pectoris-Myocardial Infarction Investigations in Japan. Journal of the American College of Cardiology. 2001 Jul:38(1):11-8
[PubMed PMID: 11451258]
[12]
Ogura R, Hiasa Y, Takahashi T, Yamaguchi K, Fujiwara K, Ohara Y, Nada T, Ogata T, Kusunoki K, Yuba K, Hosokawa S, Kishi K, Ohtani R. Specific findings of the standard 12-lead ECG in patients with 'Takotsubo' cardiomyopathy: comparison with the findings of acute anterior myocardial infarction. Circulation journal : official journal of the Japanese Circulation Society. 2003 Aug:67(8):687-90
[PubMed PMID: 12890911]
[13]
Hurtado Rendón IS, Alcivar D, Rodriguez-Escudero JP, Silver K. Acute Myocardial Infarction and Stress Cardiomyopathy Are Not Mutually Exclusive. The American journal of medicine. 2018 Feb:131(2):202-205. doi: 10.1016/j.amjmed.2017.07.039. Epub 2017 Aug 30
[PubMed PMID: 28860031]
[14]
Kassim TA, Clarke DD, Mai VQ, Clyde PW, Mohamed Shakir KM. Catecholamine-induced cardiomyopathy. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2008 Dec:14(9):1137-49
[PubMed PMID: 19158054]
[15]
Ako J, Sudhir K, Farouque HM, Honda Y, Fitzgerald PJ. Transient left ventricular dysfunction under severe stress: brain-heart relationship revisited. The American journal of medicine. 2006 Jan:119(1):10-7
[PubMed PMID: 16431176]
[16]
Dastidar AG, Frontera A, Palazzuoli A, Bucciarelli-Ducci C. TakoTsubo cardiomyopathy: unravelling the malignant consequences of a benign disease with cardiac magnetic resonance. Heart failure reviews. 2015 Jul:20(4):415-21. doi: 10.1007/s10741-015-9489-4. Epub
[PubMed PMID: 25896529]
[17]
Sharkey SW, Lesser JR, Zenovich AG, Maron MS, Lindberg J, Longe TF, Maron BJ. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation. 2005 Feb 1:111(4):472-9
[PubMed PMID: 15687136]
[18]
Desmet WJ, Adriaenssens BF, Dens JA. Apical ballooning of the left ventricle: first series in white patients. Heart (British Cardiac Society). 2003 Sep:89(9):1027-31
[PubMed PMID: 12923018]