[1]
Cole ST. Learning from the genome sequence of Mycobacterium tuberculosis H37Rv. FEBS letters. 1999 Jun 4:452(1-2):7-10
[PubMed PMID: 10376668]
Level 3 (low-level) evidence
[2]
Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE 3rd, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998 Jun 11:393(6685):537-44
[PubMed PMID: 9634230]
[3]
Lalvani A, Whitworth HS. Progress in interferon-gamma release assay development and applications: an unfolding story of translational research. Annals of translational medicine. 2019 Jul:7(Suppl 3):S128. doi: 10.21037/atm.2019.05.76. Epub
[PubMed PMID: 31576335]
[4]
MacLean E, Kohli M, Weber SF, Suresh A, Schumacher SG, Denkinger CM, Pai M. Advances in Molecular Diagnosis of Tuberculosis. Journal of clinical microbiology. 2020 Sep 22:58(10):. doi: 10.1128/JCM.01582-19. Epub 2020 Sep 22
[PubMed PMID: 32759357]
Level 3 (low-level) evidence
[5]
. WHO consolidated guidelines on tuberculosis: Module 3: Diagnosis – Tests for tuberculosis infection. 2022:():
[PubMed PMID: 36441853]
[6]
D'Ambrosio L, Centis R, Tiberi S, Tadolini M, Dalcolmo M, Rendon A, Esposito S, Migliori GB. Delamanid and bedaquiline to treat multidrug-resistant and extensively drug-resistant tuberculosis in children: a systematic review. Journal of thoracic disease. 2017 Jul:9(7):2093-2101. doi: 10.21037/jtd.2017.06.16. Epub
[PubMed PMID: 28840010]
Level 1 (high-level) evidence
[7]
Sterling TR, Njie G, Zenner D, Cohn DL, Reves R, Ahmed A, Menzies D, Horsburgh CR Jr, Crane CM, Burgos M, LoBue P, Winston CA, Belknap R. Guidelines for the Treatment of Latent Tuberculosis Infection: Recommendations from the National Tuberculosis Controllers Association and CDC, 2020. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports. 2020 Feb 14:69(1):1-11. doi: 10.15585/mmwr.rr6901a1. Epub 2020 Feb 14
[PubMed PMID: 32053584]
[8]
Bigi MM, Forrellad MA, García JS, Blanco FC, Vázquez CL, Bigi F. An update on Mycobacterium tuberculosis lipoproteins. Future microbiology. 2023 Dec:18():1381-1398. doi: 10.2217/fmb-2023-0088. Epub 2023 Nov 14
[PubMed PMID: 37962486]
[9]
Warner DF, Koch A, Mizrahi V. Diversity and disease pathogenesis in Mycobacterium tuberculosis. Trends in microbiology. 2015 Jan:23(1):14-21. doi: 10.1016/j.tim.2014.10.005. Epub 2014 Nov 10
[PubMed PMID: 25468790]
[10]
Bagcchi S. WHO's Global Tuberculosis Report 2022. The Lancet. Microbe. 2023 Jan:4(1):e20. doi: 10.1016/S2666-5247(22)00359-7. Epub 2022 Dec 12
[PubMed PMID: 36521512]
[11]
Schildknecht KR, Pratt RH, Feng PI, Price SF, Self JL. Tuberculosis - United States, 2022. MMWR. Morbidity and mortality weekly report. 2023 Mar 24:72(12):297-303. doi: 10.15585/mmwr.mm7212a1. Epub 2023 Mar 24
[PubMed PMID: 36952282]
[12]
Drain PK, Bajema KL, Dowdy D, Dheda K, Naidoo K, Schumacher SG, Ma S, Meermeier E, Lewinsohn DM, Sherman DR. Incipient and Subclinical Tuberculosis: a Clinical Review of Early Stages and Progression of Infection. Clinical microbiology reviews. 2018 Oct:31(4):. doi: 10.1128/CMR.00021-18. Epub 2018 Jul 18
[PubMed PMID: 30021818]
[13]
Lawn SD, Wood R, Wilkinson RJ. Changing concepts of "latent tuberculosis infection" in patients living with HIV infection. Clinical & developmental immunology. 2011:2011():. pii: 980594. doi: 10.1155/2011/980594. Epub 2010 Sep 26
[PubMed PMID: 20936108]
[14]
Elkington PT, Friedland JS. Permutations of time and place in tuberculosis. The Lancet. Infectious diseases. 2015 Nov:15(11):1357-60. doi: 10.1016/S1473-3099(15)00135-8. Epub 2015 Aug 28
[PubMed PMID: 26321650]
[15]
Pai M, Behr MA, Dowdy D, Dheda K, Divangahi M, Boehme CC, Ginsberg A, Swaminathan S, Spigelman M, Getahun H, Menzies D, Raviglione M. Tuberculosis. Nature reviews. Disease primers. 2016 Oct 27:2():16076. doi: 10.1038/nrdp.2016.76. Epub 2016 Oct 27
[PubMed PMID: 27784885]
[16]
Smith I. Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clinical microbiology reviews. 2003 Jul:16(3):463-96
[PubMed PMID: 12857778]
[17]
Russell DG, Cardona PJ, Kim MJ, Allain S, Altare F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nature immunology. 2009 Sep:10(9):943-8. doi: 10.1038/ni.1781. Epub 2009 Aug 19
[PubMed PMID: 19692995]
[18]
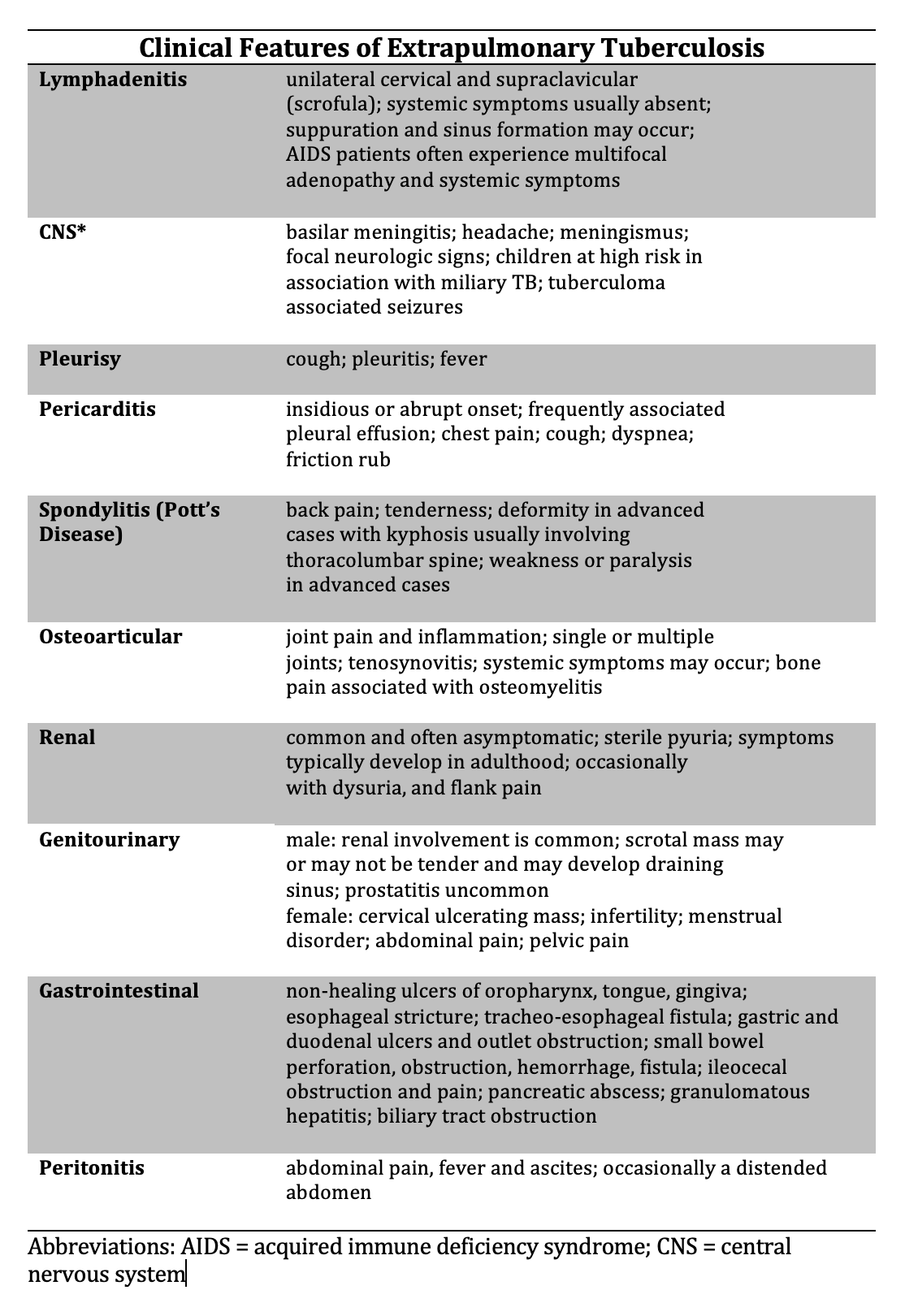
Golden MP, Vikram HR. Extrapulmonary tuberculosis: an overview. American family physician. 2005 Nov 1:72(9):1761-8
[PubMed PMID: 16300038]
Level 3 (low-level) evidence
[19]
Hoa NB, Sy DN, Nhung NV, Tiemersma EW, Borgdorff MW, Cobelens FG. National survey of tuberculosis prevalence in Viet Nam. Bulletin of the World Health Organization. 2010 Apr:88(4):273-80. doi: 10.2471/BLT.09.067801. Epub 2010 Feb 22
[PubMed PMID: 20431791]
Level 3 (low-level) evidence
[20]
Wood R, Middelkoop K, Myer L, Grant AD, Whitelaw A, Lawn SD, Kaplan G, Huebner R, McIntyre J, Bekker LG. Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. American journal of respiratory and critical care medicine. 2007 Jan 1:175(1):87-93
[PubMed PMID: 16973982]
[21]
Urbanowski ME, Ordonez AA, Ruiz-Bedoya CA, Jain SK, Bishai WR. Cavitary tuberculosis: the gateway of disease transmission. The Lancet. Infectious diseases. 2020 Jun:20(6):e117-e128. doi: 10.1016/S1473-3099(20)30148-1. Epub 2020 May 5
[PubMed PMID: 32482293]
[22]
Kendall EA, Kitonsa PJ, Nalutaaya A, Erisa KC, Mukiibi J, Nakasolya O, Isooba D, Baik Y, Robsky KO, Kato-Maeda M, Cattamanchi A, Katamba A, Dowdy DW. The Spectrum of Tuberculosis Disease in an Urban Ugandan Community and Its Health Facilities. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2021 Jun 15:72(12):e1035-e1043. doi: 10.1093/cid/ciaa1824. Epub
[PubMed PMID: 33283227]
[23]
Kashyap S, Solanki A. Challenges in endobronchial tuberculosis: from diagnosis to management. Pulmonary medicine. 2014:2014():594806. doi: 10.1155/2014/594806. Epub 2014 Aug 14
[PubMed PMID: 25197570]
[24]
He W, Tan Y, Song Z, Liu B, Wang Y, He P, Xia H, Huang F, Liu C, Zheng H, Pei S, Liu D, Ma A, Cao X, Zhao B, Ou X, Wang S, Zhao Y. Endogenous relapse and exogenous reinfection in recurrent pulmonary tuberculosis: A retrospective study revealed by whole genome sequencing. Frontiers in microbiology. 2023:14():1115295. doi: 10.3389/fmicb.2023.1115295. Epub 2023 Feb 17
[PubMed PMID: 36876077]
Level 2 (mid-level) evidence
[25]
Kwan CK, Ernst JD. HIV and tuberculosis: a deadly human syndemic. Clinical microbiology reviews. 2011 Apr:24(2):351-76. doi: 10.1128/CMR.00042-10. Epub
[PubMed PMID: 21482729]
[26]
Bruchfeld J, Correia-Neves M, Källenius G. Tuberculosis and HIV Coinfection. Cold Spring Harbor perspectives in medicine. 2015 Feb 26:5(7):a017871. doi: 10.1101/cshperspect.a017871. Epub 2015 Feb 26
[PubMed PMID: 25722472]
Level 3 (low-level) evidence
[27]
Hamada Y, Getahun H, Tadesse BT, Ford N. HIV-associated tuberculosis. International journal of STD & AIDS. 2021 Aug:32(9):780-790. doi: 10.1177/0956462421992257. Epub 2021 Feb 20
[PubMed PMID: 33612015]
[28]
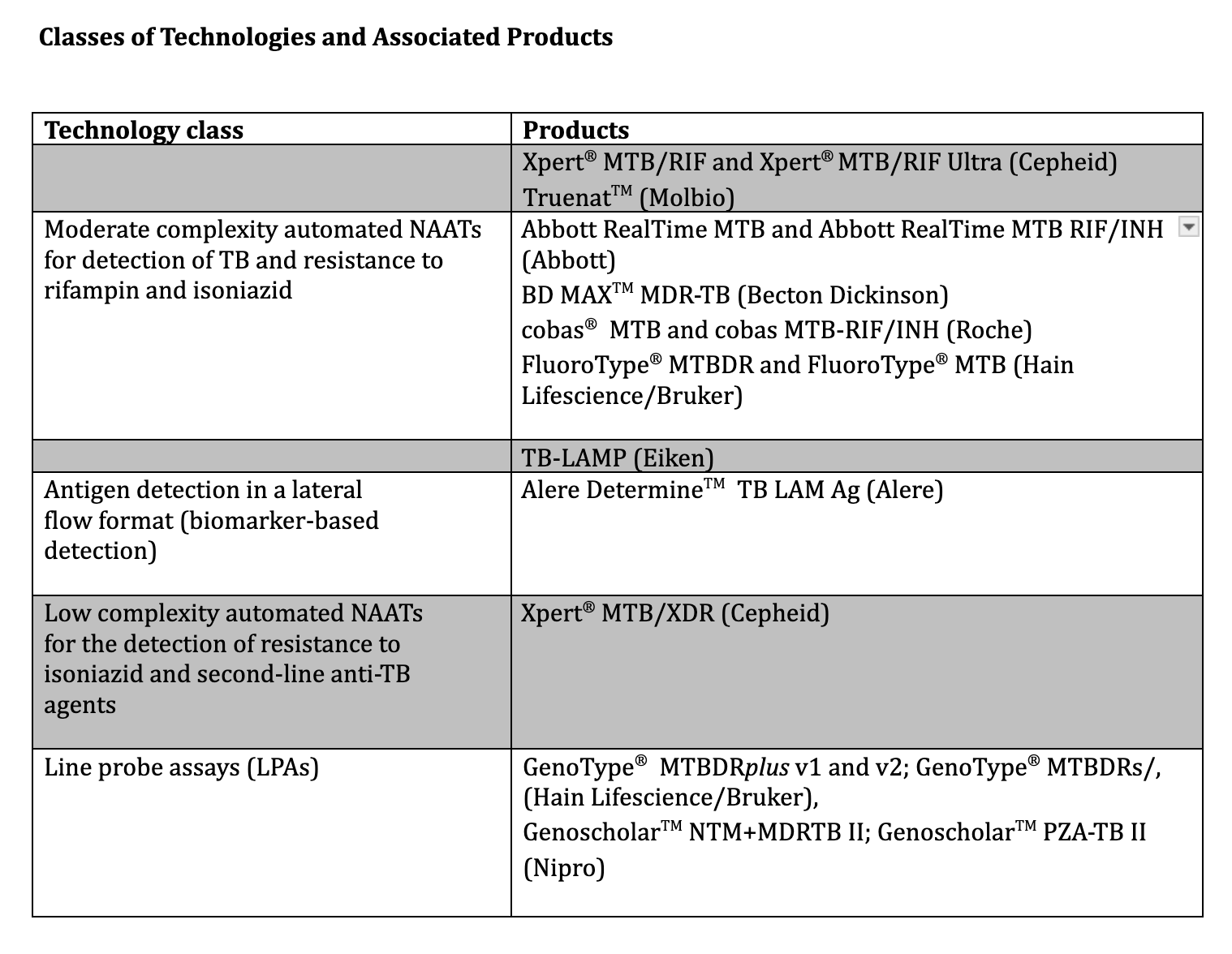
. WHO consolidated guidelines on tuberculosis: Module 3: diagnosis – rapid diagnostics for tuberculosis detection. 2021:():
[PubMed PMID: 34314130]
[29]
Getahun H, Kittikraisak W, Heilig CM, Corbett EL, Ayles H, Cain KP, Grant AD, Churchyard GJ, Kimerling M, Shah S, Lawn SD, Wood R, Maartens G, Granich R, Date AA, Varma JK. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLoS medicine. 2011 Jan 18:8(1):e1000391. doi: 10.1371/journal.pmed.1000391. Epub 2011 Jan 18
[PubMed PMID: 21267059]
Level 1 (high-level) evidence
[30]
Gadkowski LB, Stout JE. Cavitary pulmonary disease. Clinical microbiology reviews. 2008 Apr:21(2):305-33, table of contents. doi: 10.1128/CMR.00060-07. Epub
[PubMed PMID: 18400799]
[31]
Davies PD, Pai M. The diagnosis and misdiagnosis of tuberculosis. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2008 Nov:12(11):1226-34
[PubMed PMID: 18926032]
[32]
Pagaduan JV, Altawallbeh G. Advances in TB testing. Advances in clinical chemistry. 2023:115():33-62. doi: 10.1016/bs.acc.2023.03.003. Epub 2023 Mar 29
[PubMed PMID: 37673521]
Level 3 (low-level) evidence
[33]
Lewinsohn DM, Leonard MK, LoBue PA, Cohn DL, Daley CL, Desmond E, Keane J, Lewinsohn DA, Loeffler AM, Mazurek GH, O'Brien RJ, Pai M, Richeldi L, Salfinger M, Shinnick TM, Sterling TR, Warshauer DM, Woods GL. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017 Jan 15:64(2):111-115. doi: 10.1093/cid/ciw778. Epub
[PubMed PMID: 28052967]
Level 1 (high-level) evidence
[34]
Brodie D, Schluger NW. The diagnosis of tuberculosis. Clinics in chest medicine. 2005 Jun:26(2):247-71, vi
[PubMed PMID: 15837109]
[35]
Skoura E, Zumla A, Bomanji J. Imaging in tuberculosis. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2015 Mar:32():87-93. doi: 10.1016/j.ijid.2014.12.007. Epub
[PubMed PMID: 25809762]
[36]
Carranza C, Pedraza-Sanchez S, de Oyarzabal-Mendez E, Torres M. Diagnosis for Latent Tuberculosis Infection: New Alternatives. Frontiers in immunology. 2020:11():2006. doi: 10.3389/fimmu.2020.02006. Epub 2020 Sep 10
[PubMed PMID: 33013856]
[37]
Esmail H, Barry CE 3rd, Young DB, Wilkinson RJ. The ongoing challenge of latent tuberculosis. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2014:369(1645):20130437. doi: 10.1098/rstb.2013.0437. Epub 2014 May 12
[PubMed PMID: 24821923]
[38]
Menzies D, Gardiner G, Farhat M, Greenaway C, Pai M. Thinking in three dimensions: a web-based algorithm to aid the interpretation of tuberculin skin test results. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2008 May:12(5):498-505
[PubMed PMID: 18419884]
[39]
Bomanji JB, Gupta N, Gulati P, Das CJ. Imaging in tuberculosis. Cold Spring Harbor perspectives in medicine. 2015 Jan 20:5(6):. doi: 10.1101/cshperspect.a017814. Epub 2015 Jan 20
[PubMed PMID: 25605754]
Level 3 (low-level) evidence
[40]
Procop GW. Laboratory Diagnosis and Susceptibility Testing for Mycobacterium tuberculosis. Microbiology spectrum. 2016 Dec:4(6):. doi: 10.1128/microbiolspec.TNMI7-0022-2016. Epub
[PubMed PMID: 28087944]
[41]
. Automated Real-Time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert MTB/RIF Assay for the Diagnosis of Pulmonary and Extrapulmonary TB in Adults and Children: Policy Update. 2013:():
[PubMed PMID: 25473701]
[42]
da Silva MP, Cassim N, Ndlovu S, Marokane PS, Radebe M, Shapiro A, Scott LE, Stevens WS. More Than a Decade of GeneXpert(®)Mycobacterium tuberculosis/Rifampicin (Ultra) Testing in South Africa: Laboratory Insights from Twenty-Three Million Tests. Diagnostics (Basel, Switzerland). 2023 Oct 19:13(20):. doi: 10.3390/diagnostics13203253. Epub 2023 Oct 19
[PubMed PMID: 37892074]
[43]
Dorman SE, Schumacher SG, Alland D, Nabeta P, Armstrong DT, King B, Hall SL, Chakravorty S, Cirillo DM, Tukvadze N, Bablishvili N, Stevens W, Scott L, Rodrigues C, Kazi MI, Joloba M, Nakiyingi L, Nicol MP, Ghebrekristos Y, Anyango I, Murithi W, Dietze R, Lyrio Peres R, Skrahina A, Auchynka V, Chopra KK, Hanif M, Liu X, Yuan X, Boehme CC, Ellner JJ, Denkinger CM, study team. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. The Lancet. Infectious diseases. 2018 Jan:18(1):76-84. doi: 10.1016/S1473-3099(17)30691-6. Epub 2017 Nov 30
[PubMed PMID: 29198911]
[44]
Wu RI, Mark EJ, Hunt JL. Staining for acid-fast bacilli in surgical pathology: practice patterns and variations. Human pathology. 2012 Nov:43(11):1845-51. doi: 10.1016/j.humpath.2012.01.006. Epub 2012 Apr 26
[PubMed PMID: 22542129]
[45]
Mathew P, Kuo YH, Vazirani B, Eng RH, Weinstein MP. Are three sputum acid-fast bacillus smears necessary for discontinuing tuberculosis isolation? Journal of clinical microbiology. 2002 Sep:40(9):3482-4
[PubMed PMID: 12202598]
[46]
Theron G, Venter R, Calligaro G, Smith L, Limberis J, Meldau R, Chanda D, Esmail A, Peter J, Dheda K. Xpert MTB/RIF Results in Patients With Previous Tuberculosis: Can We Distinguish True From False Positive Results? Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016 Apr 15:62(8):995-1001. doi: 10.1093/cid/civ1223. Epub 2016 Feb 16
[PubMed PMID: 26908793]
[47]
Ngangue YR, Mbuli C, Neh A, Nshom E, Koudjou A, Palmer D, Ndi NN, Qin ZZ, Creswell J, Mbassa V, Vuchas C, Sander M. Diagnostic Accuracy of the Truenat MTB Plus Assay and Comparison with the Xpert MTB/RIF Assay to Detect Tuberculosis among Hospital Outpatients in Cameroon. Journal of clinical microbiology. 2022 Aug 17:60(8):e0015522. doi: 10.1128/jcm.00155-22. Epub 2022 Jul 21
[PubMed PMID: 35861529]
Level 2 (mid-level) evidence
[48]
Notomi T, Mori Y, Tomita N, Kanda H. Loop-mediated isothermal amplification (LAMP): principle, features, and future prospects. Journal of microbiology (Seoul, Korea). 2015 Jan:53(1):1-5. doi: 10.1007/s12275-015-4656-9. Epub 2015 Jan 4
[PubMed PMID: 25557475]
[49]
Bulterys MA, Wagner B, Redard-Jacot M, Suresh A, Pollock NR, Moreau E, Denkinger CM, Drain PK, Broger T. Point-Of-Care Urine LAM Tests for Tuberculosis Diagnosis: A Status Update. Journal of clinical medicine. 2019 Dec 31:9(1):. doi: 10.3390/jcm9010111. Epub 2019 Dec 31
[PubMed PMID: 31906163]
[50]
Pillay S, de Vos M, Sohn H, Ghebrekristos Y, Dolby T, Warren RM, Theron G. To Test or Not? Xpert MTB/RIF as an Alternative to Smear Microscopy to Guide Line Probe Assay Testing for Drug-Resistant Tuberculosis. Journal of clinical microbiology. 2023 Jul 20:61(7):e0001723. doi: 10.1128/jcm.00017-23. Epub 2023 Jun 27
[PubMed PMID: 37367228]
[51]
Aggarwal AN, Agarwal R, Sehgal IS, Dhooria S. Adenosine deaminase for diagnosis of tuberculous pleural effusion: A systematic review and meta-analysis. PloS one. 2019:14(3):e0213728. doi: 10.1371/journal.pone.0213728. Epub 2019 Mar 26
[PubMed PMID: 30913213]
Level 1 (high-level) evidence
[52]
Prasad MK, Kumar A, Nalini N, Kumar P, Mishra B, Lata D, Ashok C, Kumar D, Marandi S, Kumar D, Singh S, Mahajan M. Diagnostic Accuracy of Cerebrospinal Fluid (CSF) Adenosine Deaminase (ADA) for Tuberculous Meningitis (TBM) in Adults: A Systematic Review and Meta-Analysis. Cureus. 2023 Jun:15(6):e39896. doi: 10.7759/cureus.39896. Epub 2023 Jun 3
[PubMed PMID: 37404432]
Level 1 (high-level) evidence
[53]
Tao L, Ning HJ, Nie HM, Guo XY, Qin SY, Jiang HX. Diagnostic value of adenosine deaminase in ascites for tuberculosis ascites: a meta-analysis. Diagnostic microbiology and infectious disease. 2014 May:79(1):102-7. doi: 10.1016/j.diagmicrobio.2013.12.010. Epub 2013 Dec 21
[PubMed PMID: 24629577]
Level 1 (high-level) evidence
[54]
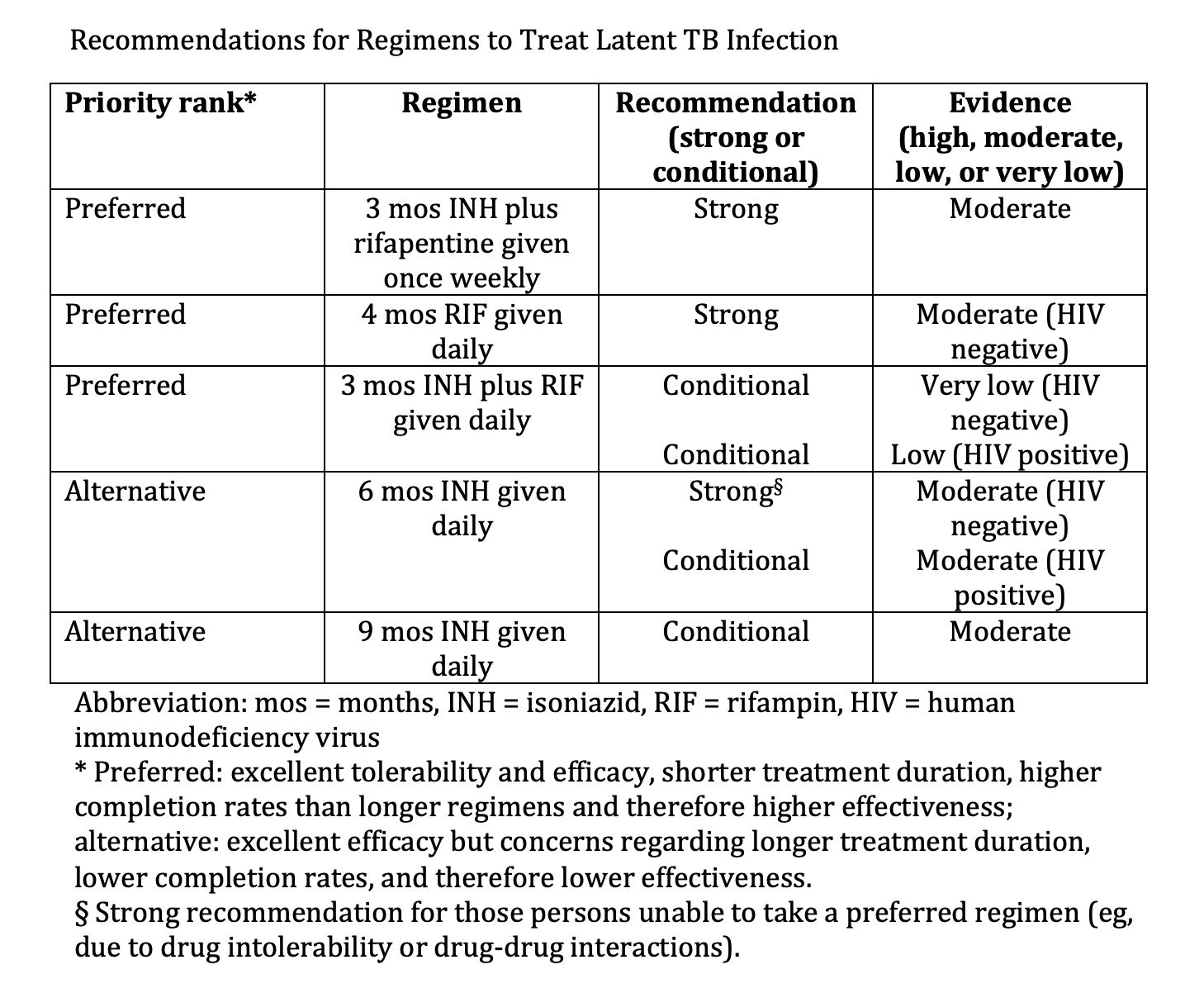
. WHO consolidated guidelines on tuberculosis: tuberculosis preventive treatment: Module 1: prevention. 2020:():
[PubMed PMID: 32186832]
[55]
Sadowski C, Belknap R, Holland DP, Moro RN, Chen MP, Wright A, Millet JP, Caylà JA, Scott NA, Borisov A, Gandhi NR. Symptoms and Systemic Drug Reactions in Persons Receiving Weekly Rifapentine Plus Isoniazid (3HP) Treatment for Latent Tuberculosis Infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2023 Jun 16:76(12):2090-2097. doi: 10.1093/cid/ciad083. Epub
[PubMed PMID: 36815322]
[56]
Kasaie P, Pennington J, Gupta A, Dowdy DW, Kendall EA. Trials underestimate the impact of preventive treatment for household contacts exposed to multidrug-resistant tuberculosis: a simulation study. medRxiv : the preprint server for health sciences. 2023 Feb 8:():. pii: 2023.02.06.23285528. doi: 10.1101/2023.02.06.23285528. Epub 2023 Feb 8
[PubMed PMID: 36798407]
[57]
Suryavanshi N, Murrill M, Gupta A, Hughes M, Hesseling A, Kim S, Naini L, Jones L, Smith B, Gupte N, Dawson R, Mave V, Meshram S, Mendoza-Ticona A, Sanchez J, Kumarasamy N, Comins K, Conradie F, Shenje J, Nerette Fontain S, Garcia-Prats A, Asmelash A, Nedsuwan S, Mohapi L, Lalloo U, Cristina Garcia Ferreira A, Okeyo E, Swindells S, Churchyard G, Shah NS. Willingness to Take Multidrug-resistant Tuberculosis (MDR-TB) Preventive Therapy Among Adult and Adolescent Household Contacts of MDR-TB Index Cases: An International Multisite Cross-sectional Study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2020 Jan 16:70(3):436-445. doi: 10.1093/cid/ciz254. Epub
[PubMed PMID: 30919881]
Level 2 (mid-level) evidence
[58]
Krishnan S, Wu X, Kim S, McIntire K, Naini L, Hughes MD, Dawson R, Mave V, Gaikwad S, Sanchez J, Mendoza-Ticona A, Gonzales P, Comins K, Shenje J, Fontain SN, Omozoarhe A, Mohapi L, Lalloo UG, Garcia Ferreira AC, Mugah C, Harrington M, Shah NS, Hesseling AC, Churchyard G, Swindells S, Gupta A, AIDS Clinical Trials Group A5300/International Maternal Pediatric Adolescent AIDS Clinical Trials I2003 Protecting Households on Exposure to Newly Diagnosed Index Multidrug-resistant Tuberculosis Patients Feasibility Study Team* (Additional study group members are listed in the Acknowledgment section). 1-Year Incidence of Tuberculosis Infection and Disease Among Household Contacts of Rifampin- and Multidrug-Resistant Tuberculosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2023 Sep 18:77(6):892-900. doi: 10.1093/cid/ciad301. Epub
[PubMed PMID: 37227925]
Level 2 (mid-level) evidence
[59]
Adjobimey M, Behr MA, Menzies D. Individualized Treatment Duration in Tuberculosis Treatment: Precision versus Simplicity. American journal of respiratory and critical care medicine. 2021 Nov 1:204(9):1013-1014. doi: 10.1164/rccm.202107-1744ED. Epub
[PubMed PMID: 34432615]
[60]
Imperial MZ, Phillips PPJ, Nahid P, Savic RM. Precision-Enhancing Risk Stratification Tools for Selecting Optimal Treatment Durations in Tuberculosis Clinical Trials. American journal of respiratory and critical care medicine. 2021 Nov 1:204(9):1086-1096. doi: 10.1164/rccm.202101-0117OC. Epub
[PubMed PMID: 34346856]
[61]
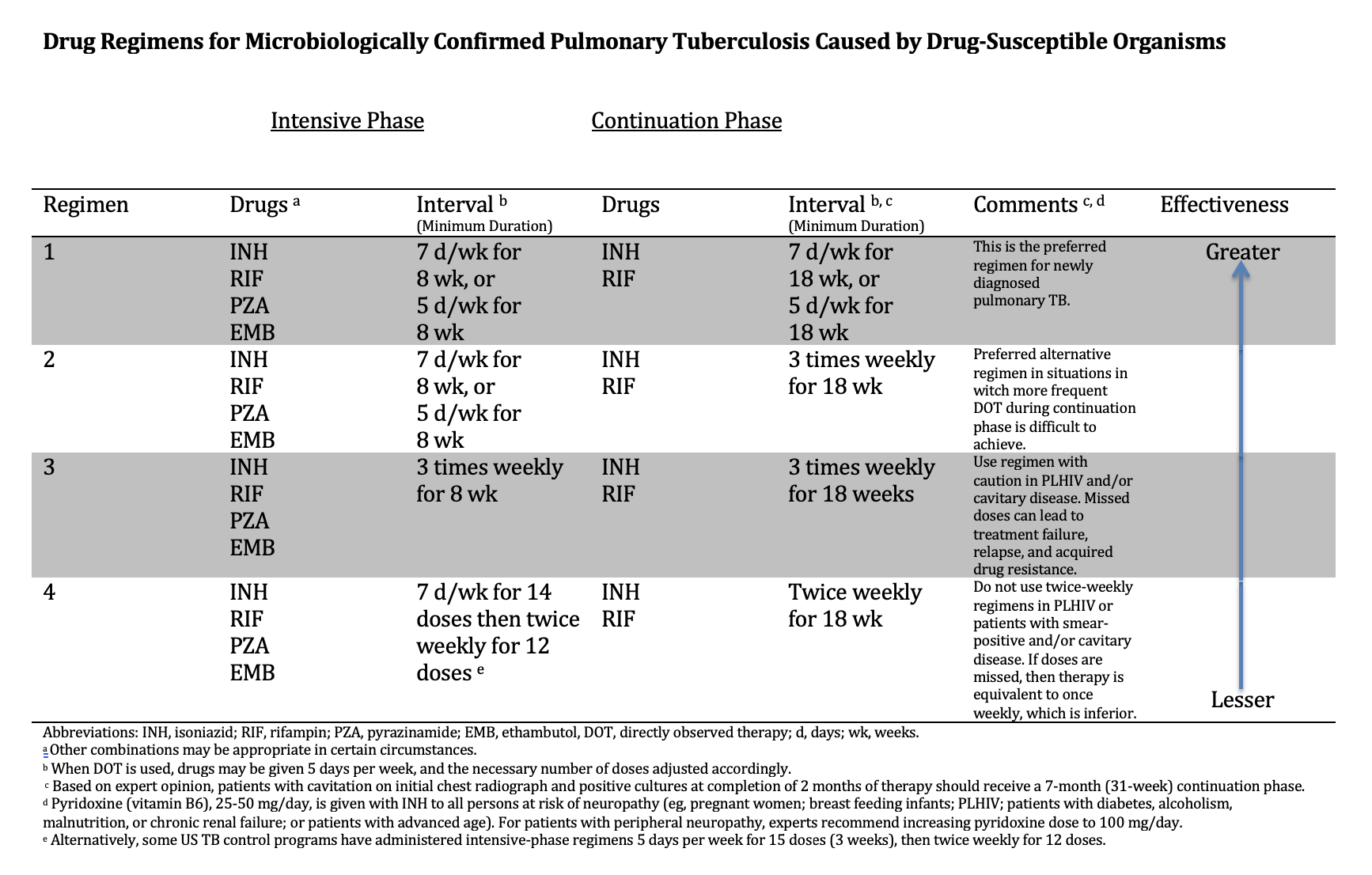
Nahid P, Dorman SE, Alipanah N, Barry PM, Brozek JL, Cattamanchi A, Chaisson LH, Chaisson RE, Daley CL, Grzemska M, Higashi JM, Ho CS, Hopewell PC, Keshavjee SA, Lienhardt C, Menzies R, Merrifield C, Narita M, O'Brien R, Peloquin CA, Raftery A, Saukkonen J, Schaaf HS, Sotgiu G, Starke JR, Migliori GB, Vernon A. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016 Oct 1:63(7):e147-e195. doi: 10.1093/cid/ciw376. Epub 2016 Aug 10
[PubMed PMID: 27516382]
Level 1 (high-level) evidence
[62]
Sotgiu G, Centis R, D'ambrosio L, Migliori GB. Tuberculosis treatment and drug regimens. Cold Spring Harbor perspectives in medicine. 2015 Jan 8:5(5):a017822. doi: 10.1101/cshperspect.a017822. Epub 2015 Jan 8
[PubMed PMID: 25573773]
Level 3 (low-level) evidence
[63]
Dorman SE, Nahid P, Kurbatova EV, Phillips PPJ, Bryant K, Dooley KE, Engle M, Goldberg SV, Phan HTT, Hakim J, Johnson JL, Lourens M, Martinson NA, Muzanyi G, Narunsky K, Nerette S, Nguyen NV, Pham TH, Pierre S, Purfield AE, Samaneka W, Savic RM, Sanne I, Scott NA, Shenje J, Sizemore E, Vernon A, Waja Z, Weiner M, Swindells S, Chaisson RE, AIDS Clinical Trials Group, Tuberculosis Trials Consortium. Four-Month Rifapentine Regimens with or without Moxifloxacin for Tuberculosis. The New England journal of medicine. 2021 May 6:384(18):1705-1718. doi: 10.1056/NEJMoa2033400. Epub
[PubMed PMID: 33951360]
[64]
Carr W, Kurbatova E, Starks A, Goswami N, Allen L, Winston C. Interim Guidance: 4-Month Rifapentine-Moxifloxacin Regimen for the Treatment of Drug-Susceptible Pulmonary Tuberculosis - United States, 2022. MMWR. Morbidity and mortality weekly report. 2022 Feb 25:71(8):285-289. doi: 10.15585/mmwr.mm7108a1. Epub 2022 Feb 25
[PubMed PMID: 35202353]
[65]
. WHO consolidated guidelines on tuberculosis: Module 4: Treatment - Drug-resistant tuberculosis treatment. 2020:():
[PubMed PMID: 32603040]
[66]
Karnan A, Jadhav U, Ghewade B, Ledwani A, Shivashankar P. A Comprehensive Review on Long vs. Short Regimens in Multidrug-Resistant Tuberculosis (MDR-TB) Under Programmatic Management of Drug-Resistant Tuberculosis (PMDT). Cureus. 2024 Jan:16(1):e52706. doi: 10.7759/cureus.52706. Epub 2024 Jan 22
[PubMed PMID: 38384625]
[67]
Stillo J, Frick M, Galarza J, Kondratyuk S, Makone A, McKenna L, Vandevelde W, Winarni P, Agbassi P. Addressing the needs of people with extensively drug-resistant TB through pre-approval access to drugs and research. Public health action. 2023 Dec:13(4):126-129. doi: 10.5588/pha.23.0033. Epub 2023 Dec 7
[PubMed PMID: 38077718]
[68]
Gupta A, Juneja S, Babawale V, Rustam Majidovich N, Ndjeka N, Thi Mai Nguyen P, Nargiza Nusratovna P, Robert Omanito D, Tiara Pakasi T, Terleeva Y, Toktogonova A, Waheed Y, Myint Z, Yanlin Z, Sahu S. Global adoption of 6-month drug-resistant TB regimens: Projected uptake by 2026. PloS one. 2024:19(1):e0296448. doi: 10.1371/journal.pone.0296448. Epub 2024 Jan 5
[PubMed PMID: 38180980]
[69]
Calligaro GL, Moodley L, Symons G, Dheda K. The medical and surgical treatment of drug-resistant tuberculosis. Journal of thoracic disease. 2014 Mar:6(3):186-95. doi: 10.3978/j.issn.2072-1439.2013.11.11. Epub
[PubMed PMID: 24624282]
[70]
Nahid P, Mase SR, Migliori GB, Sotgiu G, Bothamley GH, Brozek JL, Cattamanchi A, Cegielski JP, Chen L, Daley CL, Dalton TL, Duarte R, Fregonese F, Horsburgh CR Jr, Ahmad Khan F, Kheir F, Lan Z, Lardizabal A, Lauzardo M, Mangan JM, Marks SM, McKenna L, Menzies D, Mitnick CD, Nilsen DM, Parvez F, Peloquin CA, Raftery A, Schaaf HS, Shah NS, Starke JR, Wilson JW, Wortham JM, Chorba T, Seaworth B. Treatment of Drug-Resistant Tuberculosis. An Official ATS/CDC/ERS/IDSA Clinical Practice Guideline. American journal of respiratory and critical care medicine. 2019 Nov 15:200(10):e93-e142. doi: 10.1164/rccm.201909-1874ST. Epub
[PubMed PMID: 31729908]
Level 1 (high-level) evidence
[71]
World Health Organization. BCG vaccine: WHO position paper, February 2018 - Recommendations. Vaccine. 2018 Jun 7:36(24):3408-3410. doi: 10.1016/j.vaccine.2018.03.009. Epub 2018 Mar 30
[PubMed PMID: 29609965]
[72]
Andersen P, Doherty TM. The success and failure of BCG - implications for a novel tuberculosis vaccine. Nature reviews. Microbiology. 2005 Aug:3(8):656-62
[PubMed PMID: 16012514]
[73]
Qu M, Zhou X, Li H. BCG vaccination strategies against tuberculosis: updates and perspectives. Human vaccines & immunotherapeutics. 2021 Dec 2:17(12):5284-5295. doi: 10.1080/21645515.2021.2007711. Epub 2021 Dec 2
[PubMed PMID: 34856853]
Level 3 (low-level) evidence
[74]
Lewinsohn DM, Leonard MK, LoBue PA, Cohn DL, Daley CL, Desmond E, Keane J, Lewinsohn DA, Loeffler AM, Mazurek GH, O'Brien RJ, Pai M, Richeldi L, Salfinger M, Shinnick TM, Sterling TR, Warshauer DM, Woods GL. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017 Jan 15:64(2):e1-e33. doi: 10.1093/cid/ciw694. Epub 2016 Dec 8
[PubMed PMID: 27932390]
Level 1 (high-level) evidence
[75]
Massud A, Syed Sulaiman SA, Ahmad N, Shafqat M, Chiau Ming L, Khan AH. Frequency and Management of Adverse Drug Reactions Among Drug-Resistant Tuberculosis Patients: Analysis From a Prospective Study. Frontiers in pharmacology. 2022:13():883483. doi: 10.3389/fphar.2022.883483. Epub 2022 Jun 2
[PubMed PMID: 35747749]
[77]
Singh KP, Carvalho ACC, Centis R, D Ambrosio L, Migliori GB, Mpagama SG, Nguyen BC, Aarnoutse RE, Aleksa A, van Altena R, Bhavani PK, Bolhuis MS, Borisov S, van T Boveneind-Vrubleuskaya N, Bruchfeld J, Caminero JA, Carvalho I, Cho JG, Davies Forsman L, Dedicoat M, Dheda K, Dooley K, Furin J, García-García JM, Garcia-Prats A, Hesseling AC, Heysell SK, Hu Y, Kim HY, Manga S, Marais BJ, Margineanu I, Märtson AG, Munoz Torrico M, Nataprawira HM, Nunes E, Ong CWM, Otto-Knapp R, Palmero DJ, Peloquin CA, Rendon A, Rossato Silva D, Ruslami R, Saktiawati AMI, Santoso P, Schaaf HS, Seaworth B, Simonsson USH, Singla R, Skrahina A, Solovic I, Srivastava S, Stocker SL, Sturkenboom MGG, Svensson EM, Tadolini M, Thomas TA, Tiberi S, Trubiano J, Udwadia ZF, Verhage AR, Vu DH, Akkerman OW, Alffenaar JWC, Denholm JT. Clinical standards for the management of adverse effects during treatment for TB. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2023 Jul 1:27(7):506-519. doi: 10.5588/ijtld.23.0078. Epub
[PubMed PMID: 37353868]
[78]
McIlleron H, Meintjes G, Burman WJ, Maartens G. Complications of antiretroviral therapy in patients with tuberculosis: drug interactions, toxicity, and immune reconstitution inflammatory syndrome. The Journal of infectious diseases. 2007 Aug 15:196 Suppl 1():S63-75
[PubMed PMID: 17624828]
[79]
Nanyonga SM, Kitutu FE, Kalyango J, Frank M, Kiguba R. High Burden of Adverse Drug Reactions to Isoniazid Preventive Therapy in People Living With HIV at 3 Tertiary Hospitals in Uganda: Associated Factors. Journal of acquired immune deficiency syndromes (1999). 2022 Feb 1:89(2):215-221. doi: 10.1097/QAI.0000000000002842. Epub
[PubMed PMID: 34693930]
[80]
Lazarus G, Tjoa K, Iskandar AWB, Louisa M, Sagwa EL, Padayatchi N, Soetikno V. The effect of human immunodeficiency virus infection on adverse events during treatment of drug-resistant tuberculosis: A systematic review and meta-analysis. PloS one. 2021:16(3):e0248017. doi: 10.1371/journal.pone.0248017. Epub 2021 Mar 4
[PubMed PMID: 33662024]
Level 1 (high-level) evidence
[81]
Tiemersma EW, van der Werf MJ, Borgdorff MW, Williams BG, Nagelkerke NJ. Natural history of tuberculosis: duration and fatality of untreated pulmonary tuberculosis in HIV negative patients: a systematic review. PloS one. 2011 Apr 4:6(4):e17601. doi: 10.1371/journal.pone.0017601. Epub 2011 Apr 4
[PubMed PMID: 21483732]
Level 1 (high-level) evidence
[82]
Chakaya J, Khan M, Ntoumi F, Aklillu E, Fatima R, Mwaba P, Kapata N, Mfinanga S, Hasnain SE, Katoto PDMC, Bulabula ANH, Sam-Agudu NA, Nachega JB, Tiberi S, McHugh TD, Abubakar I, Zumla A. Global Tuberculosis Report 2020 - Reflections on the Global TB burden, treatment and prevention efforts. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2021 Dec:113 Suppl 1(Suppl 1):S7-S12. doi: 10.1016/j.ijid.2021.02.107. Epub 2021 Mar 11
[PubMed PMID: 33716195]
[83]
Nyang'wa BT, Berry C, Kazounis E, Motta I, Parpieva N, Tigay Z, Solodovnikova V, Liverko I, Moodliar R, Dodd M, Ngubane N, Rassool M, McHugh TD, Spigelman M, Moore DAJ, Ritmeijer K, du Cros P, Fielding K, TB-PRACTECAL Study Collaborators. A 24-Week, All-Oral Regimen for Rifampin-Resistant Tuberculosis. The New England journal of medicine. 2022 Dec 22:387(25):2331-2343. doi: 10.1056/NEJMoa2117166. Epub
[PubMed PMID: 36546625]
[84]
Lönnroth K, Migliori GB, Abubakar I, D'Ambrosio L, de Vries G, Diel R, Douglas P, Falzon D, Gaudreau MA, Goletti D, González Ochoa ER, LoBue P, Matteelli A, Njoo H, Solovic I, Story A, Tayeb T, van der Werf MJ, Weil D, Zellweger JP, Abdel Aziz M, Al Lawati MR, Aliberti S, Arrazola de Oñate W, Barreira D, Bhatia V, Blasi F, Bloom A, Bruchfeld J, Castelli F, Centis R, Chemtob D, Cirillo DM, Colorado A, Dadu A, Dahle UR, De Paoli L, Dias HM, Duarte R, Fattorini L, Gaga M, Getahun H, Glaziou P, Goguadze L, Del Granado M, Haas W, Järvinen A, Kwon GY, Mosca D, Nahid P, Nishikiori N, Noguer I, O'Donnell J, Pace-Asciak A, Pompa MG, Popescu GG, Robalo Cordeiro C, Rønning K, Ruhwald M, Sculier JP, Simunović A, Smith-Palmer A, Sotgiu G, Sulis G, Torres-Duque CA, Umeki K, Uplekar M, van Weezenbeek C, Vasankari T, Vitillo RJ, Voniatis C, Wanlin M, Raviglione MC. Towards tuberculosis elimination: an action framework for low-incidence countries. The European respiratory journal. 2015 Apr:45(4):928-52. doi: 10.1183/09031936.00214014. Epub
[PubMed PMID: 25792630]
[85]
O'Connell J, McNally C, Stanistreet D, de Barra E, McConkey SJ. Ending tuberculosis: the cost of missing the World Health Organization target in a low-incidence country. Irish journal of medical science. 2023 Aug:192(4):1547-1553. doi: 10.1007/s11845-022-03150-3. Epub 2022 Sep 19
[PubMed PMID: 36121600]