Introduction
The larynx splits into three distinct regions known as the supraglottis, glottis, and subglottis. Within these three regions the cartilage, neurovascular, and musculature are all intertwined to allow the larynx to function as a unit and carry out its many functions. The primary functions of the larynx are voice production, protection of the airway during respiration, and swallowing.
The article reviews the most current scientific knowledge on the anatomy and function of the laryngeal-vocal cords system, integrating with the clinic, embryology, and related conditions.
Structure and Function
Laryngeal Cartilages
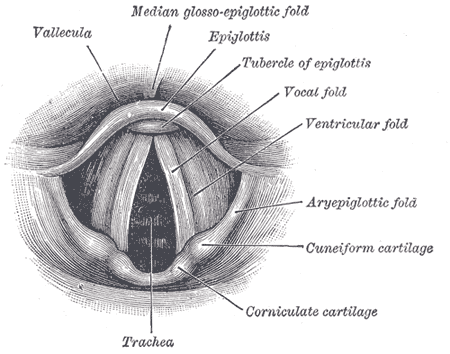
The larynx relies on cartilaginous support for its flexible, yet stable structure. The cartilages separate into two groupings. The first set of cartilages are considered to be unpaired cartilages of the larynx (known as the thyroid and cricoid cartilages). Two laminae of the thyroid cartilage come together to join anteriorly at the laryngeal prominence, popularly known as the "Adam's apple". Posteriorly, the two laminae of the thyroid cartilage remain open. The posterior aspect of each thyroid cartilage lamina extends superiorly and inferiorly forming both the superior and inferior horns. The superior horn of the thyroid cartilage makes an attachment to the hyoid bone via the thyrohyoid membrane and the lateral thyrohyoid ligament. Within this thyrohyoid membrane lies a foramen in which both the superior laryngeal vessels and the internal branch of the superior laryngeal nerve reside. The lateral thyrohyoid ligament may contain triticeous cartilage that is often mistaken for a foreign body when it calcifies. The inferior horn attaches to the cricoid cartilage via the cricothyroid membrane.
The cricoid cartilage is the only full cartilage ring within the larynx. It is composed of hyaline cartilage and often referred to as having a “signet ring” appearance. The posterior aspect of the cricoid cartilage, known as the lamina, is much wider than the anterior portion of the cartilage, referred to as the arch of the cricoid. The wider posterior portion serves as a base for the arytenoid cartilages to articulate via a ball and socket joint. Each arytenoid cartilage has two important processes that extend from it. The first being the vocal process responsible for serving as an attachment for the vocal ligament. The second process is the muscular process and serves as an attachment for intrinsic laryngeal muscles. These two processes, combined with the unique ball and socket articulation with the cricoid, allow the arytenoid to rotate within the facet causing adduction or abduction of the vocal ligament and vocal folds required for airway protection and phonation.
The second set of cartilages (arytenoid, cuneiform, and corniculate cartilages), are known as paired cartilages and lie internally within the larynx. The cuneiform and corniculate cartilages are fibroelastic cartilages and mainly function to provide rigidity to the aryepiglottic folds. The corniculate lies above and sits on the arytenoid cartilage, while the cuneiform lies within the aryepiglottic folds.
Ventricle, Folds, and Membranes
The vocal folds are now widely agreed upon and understood facets of voice because of modern histological techniques. The vocal fold comprises five layers (deep to superficial layers as follows): thyroarytenoid muscle, deep lamina propria, intermediate lamina propria, superficial lamina propria, and the squamous epithelium. The deep and intermediate lamina propria both are grouped to form the vocal ligament mentioned above. The superficial layer of the lamina propria provides a gelatinous surface upon which the vocal folds to vibrate.
The opening into the laryngeal lumen is lined by the aryepiglottic folds in which several of the cartilages lie (including the cuneiform, corniculate, and arytenoid cartilages). A common location for food to get lodged is known as the piriform sinus, and it can be found bilaterally surrounding the aryepiglottic folds.
The laryngeal ventricle is both an outpouching the laryngeal wall and potential space. It lies between the supraglottic and glottic larynx. It extends laterally as an outpouching that is known as the laryngeal saccule because of its ability to collapse upon itself. This saccule becomes important in the clinical context of saccular cysts which will be discussed further at another time. It is difficult to asses the laryngeal ventricle in its entirety when examining the patient via nasopharyngeal endoscopy; therefore, it must be in the mind of the clinician when ruling out cancer and to monitor the progression of malignancy.
Two structures within the larynx are important to prevent the spread of malignancy. The first is the quadrangular membrane. It houses the ventricular ligament. The other structure is the conus elasticus. This membrane spreads from the cricoid cartilage to the vocal ligament within the true vocal folds.
Musculature
The musculature will be discussed in detail further below in the text. However, it is important to mention how the muscles of the larynx function in phonation, swallowing, and respiration. During respiration, air flows best during abduction of the vocal folds. Therefore, it is reasonable to state that the posterior cricothyroid is solely responsible for optimal respiration. Regarding phonation, adducted vocal folds produce the best sound quality.[1] The adductors of the vocal folds, and thus those providing optimal sound quality, are the thyroarytenoid, interarytenoid, and the lateral cricoarytenoid muscles. Higher pitched phonation, however, is best when the vocal folds tense via the two bundles of the cricothyroid muscle. The pars recta, the vertically oriented bundle, attaches to the anterior portion of the cricoid and the thyroid cartilage thus causing an anterior rotation around the cricothyroid joint when it contracts. The second bundle is oriented upward and backward and is known as the pars oblique. The pars oblique contracts and subsequently displaces its attachments at the anterior surfaces of the cricoid cartilage and thyroid cartilage posteriorly. These two actions together cause increased tension and elongation of the vocal folds.[1]
Embryology
The larynx develops during the fourth week of development from both the endoderm and the mesoderm. The internal lining of the larynx originates from the endoderm while the cartilages and muscles develop from the third, fourth, and sixth pharyngeal arches. At the fourth week, an outgrowth known as the laryngotracheal groove appears from the developing foregut. This groove deepens and eventually forms the esophagotracheal septum, allowing the esophagus to lie on the dorsal side of the septum and the rest of the respiratory tract anteriorly. The groove's length continues to become the laryngotracheal diverticulum which eventually will give rise to the larynx, trachea, and lungs. The laryngeal lumen at first is obliterated due to the epithelial proliferation. However, it becomes re-canalized between weeks 7 to 10. The arches give rise to the nerves, cartilages, and musculature in the larynx. They are:
Third Branchial Arch
- Cranial nerve IX
- Greater horn of hyoid, epiglottis
Fourth Branchial Arch
- Superior Laryngeal Nerve
- Thyroid cartilage, cuneiform cartilage, epiglottis
- Cricopharyngeus muscle, cricothyroid muscle
Sixth Branchial Arch
- Recurrent Laryngeal Nerve
- Cricoid cartilage, arytenoid cartilages, corniculate cartilages
- Intrinsic musculature of the larynx
Interestingly, no part of the larynx is ossified at birth. The first to ossify is the hyoid around the second or third year of life. The teenage years are when the thyroid cartilage ossifies, while the cricoid does not ossify until the fourth decade of life.
Blood Supply and Lymphatics
The blood supply to the larynx comes from two large vessels: the external carotid artery and the subclavian artery. The external carotid artery gives off the superior thyroid artery as the first branch within the neck. From the subclavian artery originates the thyrocervical trunk, which then gives rise to the inferior thyroid artery. Each artery gives off pharyngeal arteries, which supply the larynx.
The superior and middle thyroid veins both drain into the internal jugular vein. The inferior thyroid vein drains directly into the subclavian vein. Meanwhile, lymph drainage goes to the deep cervical, paratracheal, pre-tracheal, and pre-laryngeal nodes. Interestingly, the recurrent laryngeal node is the most common site of metastasis of esophageal squamous cell carcinoma.[2]
Nerves
The primary innervation to the vocal folds comes from branches of the vagus nerve, which are the superior and inferior laryngeal nerves. The superior laryngeal nerve splits into the external laryngeal nerve and the internal laryngeal nerve approximately at the level of the greater horn of the hyoid. The internal laryngeal nerve travels through the thyrohyoid membrane with the superior laryngeal artery. The internal branch of the superior laryngeal nerve supplies all sensation to mucosa above the vocal folds, and it can be accessed rather easily from beneath the medial wall of the piriform fossa for anesthesia. The external laryngeal nerve is the source of motor innervation to the cricothyroid muscle. Its location is usually close to the superior thyroid artery as well as the superior pole of the thyroid. The several variations of its location are discussed below.
The inferior laryngeal nerve is better known as the recurrent laryngeal nerve, and it supplies all motor and sensory innervation below the vocal folds. It is known as the recurrent laryngeal nerve due to its unique course underneath the arch of the aorta on the left and underneath the subclavian artery on the right. After coursing underneath each vessel on the corresponding side, the nerve courses superiorly with the inferior thyroid artery. It eventually passes deep to the inferior constrictor muscles and enters the larynx posterior to the cricothyroid articulation. Later in this article, there is a discussion of several landmarks to help identify this nerve in surgery.
Muscles
The musculature of the larynx and surrounding structures separate into two distinct categories. The first being the external musculature that is then broken down into either depressors or elevators of the larynx based on their action. The depressors of the larynx are the sternohyoid, sternothyroid, omohyoid, and are collectively known as the "strap", or "infrahyoid", muscles. This group of muscles receives innervation from C1-C3. The elevators of the larynx are the geniohyoid, digastric, thyrohyoid, mylohyoid, and stylohyoid muscles. These are collectively known as the "suprahyoid muscles". Innervation to these muscles is more complex than the strap muscles, with innervation coming from C1, CN V3, and CN VII. The primary function of the external musculature is swallowing. The final group of external laryngeal muscles consists of the superior, middle, and inferior constrictors. These are all innervated by the pharyngeal plexus and primarily function to propel the food bolus distally.
Internal musculature is the primary group of muscles involved in phonation by either abducting or adducting the vocal folds. The sole abductor of the group is the posterior cricoarytenoid. The muscles, lateral cricoarytenoid, thyroarytenoid, interarytenoid, and cricothyroid, all act together to adduct the vocal folds. All the internal muscles of the larynx receive nerve supply from the recurrent laryngeal nerve with the exception of the cricothyroid, which is innervated by the external branch of the superior laryngeal nerve.
The exact mechanism by which the muscles function to swallow, produce voice and aid in respiration has been covered above.
Physiologic Variants
Several variations to the location to the recurrent laryngeal nerve (RLN) have been identified and will be discussed here. Chiang et al. describe variations such as extra-laryngeal branches, distorted RLN, intertwining branches of RLN between the Inferior Thyroid Artery, and non-recurrent laryngeal nerves.[3] Recognition of these variations is essential to avoid iatrogenic injury to the RLN during thyroid surgery. Extra-laryngeal branches have been reported to be present in 40-60% of patients. The most common location for extra-laryngeal branches of the RLN is at the ligament of Berry, in which both an anterior and posterior branch is seen. Distorted RLN is most often found in those patients with recurrent goiters or those presenting with a large goiter. Due to the size of these goiters, the RLN may be stretched or even pass through the capsule of the goiter increasing the risk of iatrogenic injury.[3] An article by Tang et al. studied the different variations between the inferior thyroid artery (ITA) and the RLN. They found that the RLN varies in its position bilaterally with the inferior thyroid artery. On the left, the RLN was found to be posterior to the ITA in 86.25% of cases.[4] In less than 10% of cases, it was seen anteriorly of the ITA and found to be intertwined within the branches of the ITA in 3% of cases. On the right, the RLN was found to be anterior to the ITA 75% of the time. In less than 10% of cases, is located posteriorly to the artery, while only 5% of cases it was found intertwined within the branches of the ITA.[4] A non-recurrent laryngeal nerve is much rarer than the other variations discussed above. It has never been reported on the left side on its own without other significant pathology and only been found to be present in 0.7% of patients on the right side according to a meta-analysis done by Henry et al.[5]
Surgical Considerations
The most common etiology of iatrogenic injury to the recurrent laryngeal nerve is during thyroidectomy, with a discussion of the consequences below. Therefore, surgeons must rely heavily on landmarks intraoperatively to identify and save the recurrent laryngeal nerve. Several landmarks are used intraoperatively, including the tracheoesophageal groove (TEG), tubercle of Zuckerkandl (ZT), ligament of Berry (BL), and the inferior thyroid artery (ITA). The ligament of Berry serves to connect the thyroid to the first three rings of tracheal cartilage. Studies have shown that the BL is the most reliable landmark because 78.2% of the time it coursed superficially to the RLN.[6] The next most reliable landmark to identify the recurrent laryngeal nerve is the tracheoesophageal groove. Here, it is found to lie within the TEG 63.7% of the time.[6]
Clinical Significance
Neoplasms
Recurrent Respiratory Papillomatosis (RRP) is a benign laryngeal neoplasm seen in 4 per 100,000 children and 2 per 100,000 adults.[7] The bimodal distribution of RRP subsequently categorizes the disease into both a juvenile type (presenting before age 12) and an adult type (presenting after age 12). In children, acquisition of the infection is most commonly through the vaginal canal via infected secretions from anogenital warts. In contrast, adults most often come into contact with the infection through oral sex. Regardless of the transmission, the causative virus is HPV of the papillomaviridae family. Types 6, 11, 16, 18, 31, and 33 can all cause RRP. Types 6 and 11 account for greater than 90% of cases. The presenting symptoms of RRP are dysphonia, stridor, and dyspnea. The more aggressive symptoms of RRP most commonly occur with HPV type 11. The feared complication of RRP is the involvement of the respiratory tract leading to bouts of pneumonia, atelectasis, and even hemoptysis mimicking tuberculosis. To further diagnose patients with these symptoms, chest x-rays may be performed to identify respiratory tract involvement. RRP may show up as many solid and cavitated nodules. Therefore, the utility of chest X-rays is limited. The standard imaging modality to identify these nodules within the respiratory tract is helical CT. CT findings of pulmonary RRP includes multiple centrilobular lesions that may become cystic with air-fluid levels if they become infected. They are most common in the posterior and basal regions of the lung. To date, there is no complete and curative treatment for RRP. The best mode of treatment is resection of as much of the exophytic papilloma as possible while restoring normal function of the larynx. Attempted adjuvant therapy with cidofovir has been unsuccessful.
Chondromas of the larynx and granular cell tumor are two other benign neoplasms that may affect the vocal folds. Chondromas are rare neoplasms most commonly found in men that most often arise from the lamina of the cricoid. Over 85% of chondromas occur on the cricoid, thyroid or arytenoid cartilages.[8] As discussed above, the musculature used in phonation attaches to all three cartilages. Due to the location and relationship with intra-laryngeal musculature, hoarseness is the most common complaint observed in patients presenting with chondromas. Depending on the site of the chondroma, patients may also present with dyspnea and dysphagia. Diagnosis of chondromas is usually accomplished by wedge biopsy and CT of the neck to see the extent of the chondroma. Treatment is complete resection. Granular cell tumors go by different names including granular cell myoblastomas and Abrikossoff tumors. These tumors most likely originate from Schwann cells, and only about 10% arise in the larynx.[9] When found in the larynx, granular cell tumors occur either on the true vocal fold or arytenoids. These tumors may sometimes be confused with squamous cell carcinoma due to pseudo-epitheliomatous hyperplasia visualized histologically. Cytoplasmic eosinophilic granules that stain PAS positive are also a possibility. These tumors are also S-100 positive. Most patients present with acute hoarseness of sudden onset. Treatment is by complete resection.
Reinke’s Edema (Polypoid Corditis)
The superficial lamina propria is also known as Reinke’s Space, and it is composed of loose connective tissue. Due to the makeup of Reinke’s space, it is susceptible to fluid accumulation due to chronic irritation and inflammation secondary to smoking. Irritation and inflammation eventually lead to the formation of polyps on vocal folds contributing to difficulty in the inherent vibration seen during phonation. Thus, patients will present with altered voice, more commonly a deeper voice. Women will often present complaining they sound like a man. The mainstay of treatment is first to treat the underlying disease that led to polypoid corditis such as hypothyroidism, vocal abuse, laryngopharyngeal reflux or smoking. Surgical resection is an option for those that still do not improve.
Sulcus Vocalis
When the damage to the superficial lamina propria is irreversible, it is possible that the formation of a scar or sulcus vocalis forms due to the loss of viscoelasticity. This theory is still open to debate, as a definitive explanation for the development of sulcus vocalis does not yet exist. Other theories include congenital development from branchial arch anomalies and other acquired etiologies such as laryngeal cancer or trauma.[10] Currently, treatment of sulcus vocalis aims at improving mucosal wave vibration through phonomicrosurgery or inserting vocal fold implants to prevent glottic insufficiency. Conservative measures may also be indicated in sulcus vocalis.
Benign Lesions
There are several types of benign lesions encountered on the vocal fold. Among these benign lesions are vocal fold nodules. By definition, these lesions are always found on bilateral vocal cords and often seen in young women or children. The mechanism behind the development of these nodules is usually due to repetitive trauma resulting in inflammatory changes in the vocal cords. Initially, vocal fold nodules start as acute vascular vocal fold lesions that appear erythematous and edematous. Eventually, with chronic abuse, they become fibrotic and thickened white lesions. Data shows women and children have a higher incidence of vocal fold polyps because they have relatively higher frequency voices which in theory results in increased trauma caused by the repetitive collision of the vocal folds.[11] The first line therapy for these lesions is voice therapy and other conservative measures including speech therapy, smoking cessation, and humidification of the folds.
In comparison to vocal fold nodules, vocal fold polyps can be either unilateral or bilateral. These polyps are exophytic lesions most often seen at the anterior free edge of the vocal cords. Initially, the polyps can develop as translucent mucoid polyps from inflammation and chronic irritation much like vocal fold nodules. Some polyps may go on to undergo neovascularization and have a risk of hemorrhagic rupture into the vocal folds. The primary treatment of vocal fold polyps is similar conservative treatments as described above.
Cysts
Several cysts occur within the vocal folds and can secondarily disrupt the natural voice production. Vocal fold cysts are subepithelial in origin and can be either unilateral or bilateral. These cysts are usually secondary to chronic irritation and may also cause reactive changes on the opposite fold. Definitive therapy is micro flap resection. An acquired subglottic cyst presents with post-intubation stridor due to obstruction of the mucous glands by an ET tube. Saccular cysts were briefly referenced earlier. Due to the many mucous filled glands in the ventricular saccule, these cysts are most often mucous filled secondary to obstruction of the mucous glands from prolonged intubation. These cysts do not communicate with the laryngeal cavity, while a laryngocele does. Laryngocele is a dilation of air of the laryngeal ventricle and is more common in adults, compared to saccular cysts which are more common in children. Cysts can be either internal or external. Internal cysts lie within the thyroid cartilage and most often present with hoarseness. External cysts produce a laryngocele sac that protrudes the thyrohyoid membrane thus presenting with a compressible mass that will increase in size with increased pressure within the larynx.
Vocal Fold Paralysis
Many etiologies of vocal fold paralysis are known, but the most common of those include neural denervation of the ipsilateral vagus or recurrent laryngeal nerve and mechanical fixation of intra-laryngeal cartilage. Initial presenting symptoms of those with unilateral paralysis include hoarseness, aspiration, and even complete asymptomatic symptomatology. Those with bilateral paralysis will also have stridor. Initial workup includes both laryngoscopy and stroboscopy to asses the function of the folds. On laryngoscopy, pooling of secretions, anterior displacement of the arytenoids, and a paramedian vocal fold during phonation are the presenting features of vocal fold paralysis. Meanwhile, stroboscopy will show a glottic gap due to the insufficient vocal folds inability to close completely during phonation. Also, higher amplitude asymmetry values are often seen in vocal folds with paralysis.[12] Another useful clinical test is laryngeal electromyography (EMG). EMG allows the differentiation between mechanical and neurological causes of vocal fold immobility as well as the time in which the vocal fold is denervated or reinnervated. In cases of vocal fold paralysis from mechanical etiologies, the EMG will be normal, with multiple action potentials from motor units that will develop into a normal pattern. Those with denervation injury, potentials are fibrillated with spontaneous positive waves while those that undergo reinnervation of the vocal fold show polyphasic motor units. This difference is significant because often the EMG will show reinnervation changes far before motor functionality of the vocal fold returns. When mechanical and neural factors are absent, secondary etiologies must be explored. Other etiologies that are less common of vocal fold paralysis include infections such as Lyme disease, syphilis, TB, and EBV, malignancy, and toxins (such as lead, arsenic, quinine, and streptomycin). Other modalities used to determine the underlying cause of vocal fold paralysis include chest x-rays, modified barium swallow, an esophagram, and labs (including CBC, a treponemal test, and Lyme titers). CT of the skull base down through the course of the vagus nerve may also be done to determine lesions of the vagus nerve.
Several management strategies are used to help patients with vocal fold paralysis. Approaches for unilateral vocal fold paralysis involve medialization of the vocal fold in situations where there is glottic insufficiency or to temporarily provide relief until reinnervation of the vocal fold occurs. Vocal fold medialization is done either by injection or thyroplasty and often arytenoid adduction accompanies thyroplasty medialization. Frequently, temporary medialization by injection is the first step in management through augmentation of the paralyzed vocal fold through injection of a variety of different materials. Materials used for temporary medialization are bovine gelatin, collagen products, hyaluronic acid, and carboxymethylcellulose.[13] Temporary medialization is a common choice when recovery of voice production is past six months or permanent. EMG is used to help further guide prognosis in such cases. When the prognosis exceeds six months or becomes permanent vocal fold paresis, permanent injections with calcium hydroxyapatite and autologous fat can augment the vocal fold. Historically, polytetrafluoroethylene (PTFE) paste was also used as a permanent injection material. However, its use has decreased due to the risk of granuloma formation.
Medialization via thyroplasty involves the injection of silicone elastomer, stretched PTFE, or hydroxyapatite, into the para-glottic space which then subsequently medializes the vocal fold through mass effect (known as a Type I thyroplasty). Advantages to this kind of medialization include the ease of reversibility and the maintenance of the physiological mucosal wave necessary to produce phonation. In a retrospective study done by Watanabe et al. they determined the most likely complication of medialization thyroplasty to be extrusion into the lumen, followed by granuloma formation.[14] Often, arytenoid adduction is done at the time of thyroplasty to reposition the arytenoids into a more baseline physiologic position to achieve maximum efficiency when producing voice. The indication to add arytenoid adduction procedures includes a large triangular glottic defect. The final method of management for unilateral vocal fold paresis involves a reinnervation procedure using the ansa cervicalis as a graft. This procedure maintains tone in the vocal fold, however, does not produce physiologic motion to re-establish voice, often needing augmentation to supplement reinnervation.
In the acute setting of bilateral vocal fold paralysis, the first step is often to perform a suture lateralization to maintain airway competency while awaiting the return of the inherent motion of either or both sides of the vocal fold, which once achieved, unilateral or bilateral laser cordotomy may be employed in combination with arytenoidectomy to restore airway patency. This is usually done stepwise until the perfect balance between voice production and airway patency is achieved. In those cases of bilateral paramedian paralyzed vocal folds, augmentation and injection can be used as described above to restore the voice.
Other Issues
When the respiratory musculature, for different reasons, does not produce sufficient force to emit sounds, the laryngeal system must compensate with muscle fatigue. The voice loses quality, and the vocal cords can undergo significant changes. A study of 29 patients showed that rehabilitation exercises to increase the expiratory force could decrease this gap (between the diaphragm and the vocal cords), improving the emitted voice.[15]
A recent meta-analysis on the physiotherapy and recovery of speech disorders show agreement on the effectiveness of inserting a rehabilitation process to improve the phonetics and strength of the sounds emitted. The therapist's action is not only directed on the respiratory system, but also in correcting the posture of the trunk, shoulders, and cervical tract. Laryngeal mechanoreceptors regulate the tone of the vocal cords based on the posture taken by the person. Correct posture is equivalent to a better tone of the vocal cords and an emission of high-quality sounds.[16]