Introduction
The spinal nerves emanate from the spinal cord as pairs of nerves composed of both sensory and motor fibers that function as the intermediary between the central nervous system (CNS) and the periphery. These mixed nerves collectively transmit sensory, motor, and autonomic impulses between the spinal cord and the rest of the body. In total, there are 31 pairs of spinal nerves grouped regionally by spinal region. More specifically, there are eight cervical nerve pairs (C1-C8), twelve thoracic nerve pairs (T1-T12), five lumbar nerve pairs (L1-L5), 5 sacral (S1-S5), and a single coccygeal nerve pair. While the nerves branch directly from the spinal cord and the central nervous system, the spinal nerves classify as a part of the peripheral nervous system.
Structure and Function
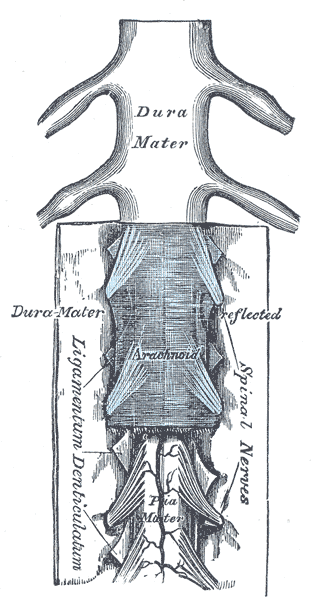
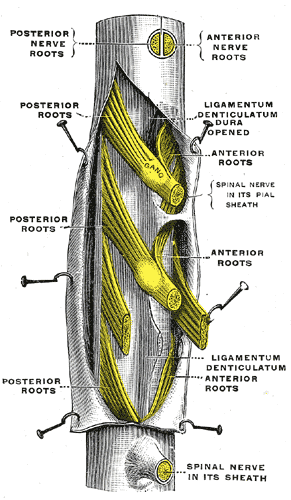
Spinal nerves are mixed nerves that interact directly with the spinal cord to modulate motor and sensory information from the body’s periphery. Each nerve forms from nerve fibers, known as fila radicularia, extending from the posterior (dorsal) and anterior (ventral) roots of the spinal cord. The roots connect via interneurons. Grossly, the root fibers join together within the intervertebral foramina to form a spinal nerve.
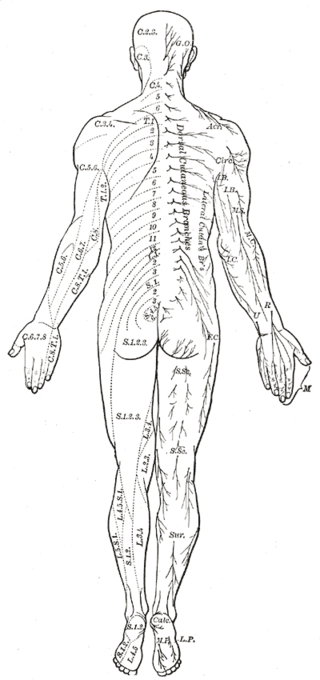
The dorsal root is composed of afferent sensory axons that transmit visceral and somatic sensory information from peripheral receptors back to the central nervous system. Areas of cutaneous sensory innervation by specific spinal nerves are mappable in an organized fashion in regions across the body known as dermatomes. While the dorsal root is synapses at the dorsal horn of the spinal cord, the sensory cell bodies of these pseudounipolar neurons are in the dorsal root ganglion, an oval enlargement just outside the cord. To communicate with the effector division of the spinal nerve, the dorsal root will synapse on an interneuron in the cord’s gray matter as part of the motor reflex arc. However, fibers of the dorsal root may instead ascend through multiple pathways in the spinal cord to provide sensory information to the thalamus.[1][2]
Meanwhile, the ventral (anterior) root bundle is responsible for transmitting somatic motor output from the brain and spinal cord to the body’s skeletal muscles. Cell bodies of the efferent motor fibers get housed in the anterior horn of the spinal cord, specifically the anterior and lateral gray columns.[2] All the muscles innervated by a particular spinal nerve are known as the nerve’s myotome. There are robust enlargements of the cord at the cranial and lumbosacral regions as these areas are responsible for a significant degree of skeletal muscle innervation of the upper and lower extremities, respectively.[2][3][4] The ventral root is composed of both alpha and gamma motor neuron axons, which supply extrafusal and intrafusal striated muscle, respectively.
The spinal nerve exits the vertebral canal through the intervertebral foramina as a single fascicle of mixed nerve fibers. The only exception to this rule is at the C1 spinal level, where the C1 spinal nerve exits the column between the occiput and the atlas (C1). Because the spinal cord does not track the entire length of the vertebral column, spinal nerves in the more cranial regions exit the spinal cord horizontally before directly passing into the periphery. Meanwhile, spinal nerves caudal to the terminus of the spinal cord (typically at the L1 or L2 vertebral level) must travel inferiorly in the column before exiting. These rootlets are seen anatomically without the spinal cord and are called the cauda equina. Tracking inferiorly from C1, all spinal nerves above C7 exit the vertebral column above their associated vertebrae. Spinal nerve C8 exits the intervertebral foramina between C7 and T1. All remaining spinal nerves depart the vertebra column caudally to their respective vertebrae.[2][5]
After exiting the vertebral column, the bundled spinal nerve divides into ventral and dorsal rami. Both rami contain mixed fibers that provide both sensory and motor innervation. The dorsal ramus is typically smaller than its ventral counterpart and consists of a medial and a lateral branch; however, some literature may also refer to a third, intermediate branch.[2][6] The branches of the dorsal rami are responsible for innervating paraspinous muscles (the nerve’s myotome) and regions of skin (the nerve’s dermatome) related to the ramus’ vertebral level. Responsibility of the lateral and medial branches of dorsal rami in thoracic spinal nerves varies based on vertebral level. Superior to T6, the medial branch provides muscular and cutaneous innervation, and the lateral branch solely provides solely muscular innervation. The opposite is true for these branches caudally to T6. Meanwhile, ventral rami are much more robust in size and function in comparison to their dorsal counterpart. The ventral rami provide the spinal contributions to all major neural plexuses. As such, they are responsible for the majority of the body’s sensorimotor innervation.[2][6]
Also of note, preganglionic cells within the autonomic nervous system (ANS) are closely associated with the sensorimotor outflow tracts of spinal nerves. The goal of the ANS is to control visceral motor tone involuntarily. Autonomic central neuronal bodies originate in the regions of the cord’s lateral horn of the cord’s gray matter, specifically in the intermediolateral nucleus. In the thoracic and upper lumbar regions (T1 to L2), these neurons give rise to preganglionic sympathetic axons that exit with somatic motor axons through the spinal cord’s ventral (anterior) root. The preganglionic fibers travel within white rami communicantes to paravertebral ganglia within the sympathetic trunk. From the ganglia, the sympathetic tone can undergo modulation in smooth and cardiac muscle, glands, and immune system cells via a series of gray rami communicantes and postganglionic fibers.[2][3]
In the parasympathetic portion of the ANS, preganglionic cells originate in the craniosacral system. Included in this system are cranial nerves III, VII, IX, and X, as well as S2 to S4 of the sacral spinal cord. The preganglionic fibers of the parasympathetic system are much longer than their sympathetic counterparts. Rather than terminating abruptly at paravertebral ganglion, parasympathetic preganglionic nerves carry impulses to peripherally located visceral ganglia that are anatomically associated with the nerve’s target tissue.[3]
Cervical Plexus
Much of the upper half of the cervical nerves comprise the cervical plexus, specifically from the anterior rami of C1 to C5. Its sensory fibers provide cutaneous innervation to the scalp, neck, chest, and axilla, as well as proprioceptive innervation of the same area via the lesser occipital nerve (C2 to C3), the great auricular nerve (C2, C3), transverse cervical nerve (C2, C3), and the supraclavicular nerve (C3, C4). The motor branches of the cervical plexus promote movement of the neck and innervation of the diaphragm. The cervical plexus also provides motor innervation to the infrahyoid muscles and the sternocleidomastoid via the ansa cervicalis (C1 to C3). Additionally, the anterior rami of the C3 and C4 loop and combine with outputs from C5 to innervate the phrenic nerve to regulate respiration.[2][7]
Brachial Plexus
The brachial plexus is a highly complex network of nerves formed from the ventral roots C5-C8, with additional contribution from T1. The five nerve roots coalesce into trunks, divisions, cords, and branches that innervate about 50 muscles and skin in the upper extremities and pectoral region.[8] C5-C6 form the superior trunk, C7 extends as the middle trunk, and C8 and T1 join to create the inferior trunk. Several significant mixed nerves extend from the brachial plexus, including the axillary (C5, C6), musculocutaneous (C5, C6) radial (C6-C8), median (C5-T1), and ulnar (C8, T1) nerves. Meanwhile, there are a handful of other nerves in the plexus that are solely muscular or cutaneous sensory nerves.[4] The nerve fibers of the brachial plexus are also accompanied by autonomic fibers, particularly from T1, that regulate vasomotor control of the upper extremity and trunk.[8]
Thoracic Nerves
There are 12 pairs of spinal nerves in the thoracic spine, one for each corresponding spinal segment. The thoracic nerves are responsible for cutaneous innervation of the skin, musculoskeletal system, and viscera. Peripheral and visceral motor fibers also innervate the musculature of the thorax and deep back, abdominal wall, and gut. Because much of the sympathetic trunk arises from the thoracic spine, pre- and postganglionic sympathetic fibers are also found coursing with spinal nerves in this region.[3]
Lumbosacral Plexus
The lumbar and sacral plexuses share nerve root overlap and are thus often referred to simply as the lumbosacral plexus. The combined plexus contains roughly 200000 axons and provides all sensory and motor innervation to the lower extremity, with some additional innervation of the abdominal wall. The combined plexus gives rise to six sensory nerves and six more sensorimotor branches.[8]
Despite the connection via the lumbosacral trunk, the two plexuses exist as separate bundles anatomically. The lumbar plexus arises from primary branches of the anterior roots of spinal nerves L1-L4. It lies superior to the pelvic rim and passes through the psoas muscle. Arising from the L1 and L2 roots of the plexus are the iliohypogastric, ilioinguinal, and genitofemoral nerves. The lateral femoral cutaneous nerve receives a contribution from L2 and L3, while the femoral and obturator nerves both branch from L3 and L4. The nerves originating from L1 and L2 innervate the transverse abdominal and anterior internal oblique muscles and provide sensory innervation to that same region, in addition to the sex organs. Meanwhile, the nerves of L3 and L4 are responsible for generating flexion and adduction of the thigh and leg extension. These nerves also provide cutaneous sensory innervation to the thigh and medial leg.[3][8] Preganglionic sympathetic fibers originating in the lateral horn of the spinal cord’s gray matter are also located at spinal levels L1 and L2.[3]
The main branches of the sacral plexus originate below the pelvic rim and are housed in the pelvic girdle.[8] It includes the superior gluteal nerve (L4-S1), the inferior gluteal nerve (L4-S1), the posterior femoral cutaneous nerve (S1-S3), and the sciatic nerve (L4-S3). The sciatic nerve is unique in that can be discretely mapped into its tibial (L4-S2) and common peroneal (L4-S1) branches. The pudendal nerve may also branch from the common sciatic nerve. The gluteal and common sciatic nerves are responsible for motor innervation of the gluteal region and posterior thigh to generate movement of the hip in all directions as well as flexion of the knee. The tibial and common peroneal nerves also dictate all motor innervation of the leg, ankle, foot, and toes. These two nerves also provide sensory innervation of the posterolateral half of the leg and the foot. The posterior femoral cutaneous nerve is solely responsible for gluteal and perineal sensory innervation.[3][8] It is also important to specify that preganglionic parasympathetic fibers originating in the sacral region are between S2 and S4.[3]
Embryology
Brain and spinal cord formation arise during the embryonic process of neurulation. During the fourth week of development, the neural plate, which arises from neural ectoderm, thickens, and folds along the dorsal midline. After folding, the neural tube then undergoes sequential differentiation, organization, and then closure in an extremely complex and regulated process to complete the primary neural tube, much of which is regulated by the notochord. The neural tube eventually develops into the primitive central nervous system, separating into its ventral and dorsal components.[9]
Meanwhile, neurons in the peripheral nervous system arise from neural crest cells after undergoing epithelial to mesenchymal transitioning. Motoneurons being among the first fibers of the peripheral system to develop.[9][10] The dermatomes and myotomes, which receive innervation from individual spinal nerves, develop segmentally from somites. Somites develop from the paraxial mesoderm.[11]
Blood Supply and Lymphatics
The spinal cord receives both longitudinal and segmental blood supply. The vasculature is highly complex and anastomotic, which ensures adequate delivery to the entire structure. Longitudinally, the anterior spinal artery (ASA) and a pair of posterior spinal arteries (PSAs) are the predominant supply of the spinal cord. The three arteries branch from the distal vertebral arteries at the base of the skull. The three vessels connect circumferentially around the cord as the vaso-corona to supply the entire periphery of the cord.[12] Numerous radicular arteries are also arranged segmentally, which provide additional blood flow to the entire vertebral column. Many of these are branches of segmental intercostal and lumbar arteries.[12][13]
Physiologic Variants
There is ample documentation regarding anatomic variations of peripheral and spinal nerves in the medical literature. Most common variants involve motor neural plexuses, where roots from multiple spinal nerves coalesce into trunks with numerous branches and outflow tracts. Anomalies in the brachial and lumbosacral plexuses often present as bifurcations, early splits, or complete absences of minor branches. These differences become particularly important in cases that require the use of a local anesthetic.[14][15]
Surgical Considerations
Several clinical considerations apply to spinal nerve anatomy. As previously mentioned, nerves exiting the cervical spine course horizontally from the spinal cord, exiting the intervertebral foramen. On the other hand, since the spinal cord usually extends caudally to the level of the L1 or L2 vertebral levels, the more caudal nerve roots travel in a somewhat vertical fashion caudally until exiting at their respective intervertebral foramen. For this reason, In the cervical spine, any type of disc herniation would result in symptoms and or neurologic deficits at the nerve root exiting at that specific level. For example, a C5-C6 right-sided disc herniation would cause symptoms and or neurologic deficits in the C6 distribution regardless of the disc herniation being far lateral or paracentral.
In contrast, in the lumbar spine, the involved nerve root would depend on the type of disc herniation. Far lateral disc herniations will present symptoms in the distribution of the exiting nerve root, whereas a paracentral disc herniation would present symptoms in the distribution of the traversing nerve root at that level. For example, a far lateral disc herniation at the level of L4-L5 would present symptoms in an L4 distribution, whereas a paracentral disc herniation would present symptoms in an L5 distribution.
Clinical Significance
Spinal nerves own a vital role in medicine, operating as the relay axons between the central and peripheral nervous systems. They play a role in a full degree of clinical practice.
Cutaneous innervation of the periphery maps into dermatomes, which are areas of skin supplied by nerves arising from a single spinal root. Understanding the topographical distribution is central to the diagnosis of a wide range of pathology. Herpes zoster, a reactivation of the varicella-zoster virus within the dorsal root ganglion of a spinal nerve, presents as a prominent and painful red rash within a specific dermatome. Meanwhile, peripheral radiculopathy is more readily diagnosed if the affected nerve, and its subsequent spinal contributions, can be identified.
Similarly, myotomes are the motor equivalent of dermatomes. The myotome refers to the group of skeletal muscles that a single spinal nerve innervates. While dermatomal distribution exhibits discreet separation by spinal nerves, there is a notable amount of overlap in motor innervation. Most prominent peripheral nerves originate from multiple spinal roots, some of which may arise from an adjacent but separate spinal region. Similarly, individual muscles often share multiple sources of spinal nerve innervation. Thus, myotomes are instead grouped by musculature action rather than strictly by anatomical location. Applying this concept in the clinical setting, motor deficits of a specific action in many muscles can help identify the presence of neurological motor deficit.