Continuing Education Activity
Healthcare professionals should be knowledgeable about chemical burns from exposure to acids (pH less than 7), alkalis (pH greater than 7), and irritants to recognize, manage and care for these common types of injury. Chemical burns are the result of exposure to a variety of substances commonly found in the home, workplace, and surrounding environment. The burn may be obvious, for example, from a direct spill or other exposure, or more covert, especially in children. Chemical burns can cause short-term, long-term, and lifelong health problems, especially if undertreated. Occasionally, they can result in premature death, especially if ingested in an attempt to self-harm. This activity reviews the pathophysiology and presentation of chemical burns and highlights the role of the interprofessional team in its management.
Objectives:
- Summarize the various possible causes of chemical burns.
- Describe the physical exam of a patient with a suspected chemical burn.
- Review the management options available for patients presenting with chemical burns.
- Explain the importance of interprofessional team strategies for improving care coordination and communication to aid in prompt diagnosis of chemical burns and improving outcomes in patients diagnosed with the condition.
Introduction
Healthcare professionals should understand chemical burns from exposure to acids (pH less than 7), alkalis (pH greater than 7), and irritants to recognize, manage and care for these common types of injury.[1][2][3]
Etiology
Chemical burns are the result of exposure to a variety of substances commonly found at home, the workplace, and the surrounding environment. The burn may be obvious, for example, from a direct spill or other exposure, or more covert, especially in children. Chemical burns can cause short-term, long-term, and lifelong health problems, especially if undertreated. Occasionally, they can result in premature death, especially if ingested in an attempt to self-harm.[4][5]
Common causes of chemical burns include the following:
- Acids: Sulfuric, nitric, hydrofluoric, hydrochloric, acetic acid, formic, phosphoric, phenols, and chloroacetic acid
- Bases: Sodium and potassium hydroxide, calcium hydroxide, sodium and calcium hypochlorite, ammonia, phosphates, silicated, sodium carbonate, lithium hydride
- Oxidants: Bleaches like chlorites used in the home, peroxides, chromates, magnates
- Miscellaneous: White phosphorus, metals, hair coloring agents, airbag injuries
- Vesicants like mustard gas
Epidemiology
Chemical burns occur commonly in children who explore at their "cruiser" level. Many households keep toxic chemicals under the sink or in other low-lying locations where a child may access them. Additionally, in the workplace or home environment, an individual may contact one or more chemicals that have the potential to cause external or internal injury, either because of unawareness of exposure or casual contact.[6][7]
In the last few years in the United Kingdom, there have been many caustic chemical assaults on women.
Children tend to suffer chemical injuries in the home; whereas, adults suffer chemical injuries in the workplace.
Pathophysiology
Chemical burns cause damage as a result of irritant properties, acidity/alkalinity, concentration, form, amount of contact, the length of exposure, and location of contact. For example, contact with a mucosal surface such as the eye is likely to cause earlier and more extensive damage than contact with intact skin where there may be some barrier protection. After inadvertent or intentional ingestion, there will be prompt contact with the mucosal surface and both direct and absorptive toxicity.
Toxicokinetics
After exposure to an alkaline agent, the -OH moiety causes injury due to liquefaction necrosis, which leads to often irreversible changes in the protein matrix. Additionally, there is vascular damage that can create a local or systemic effect.
Acidic agents cause coagulation necrosis, which leads to cytotoxicity. Additionally, there are mucosal or skin changes that may prevent further toxicity and limit absorption.
Overall, alkaline agents are more toxic than acidic agents, due to the irreversible changes in protein and tissue damage.
History and Physical
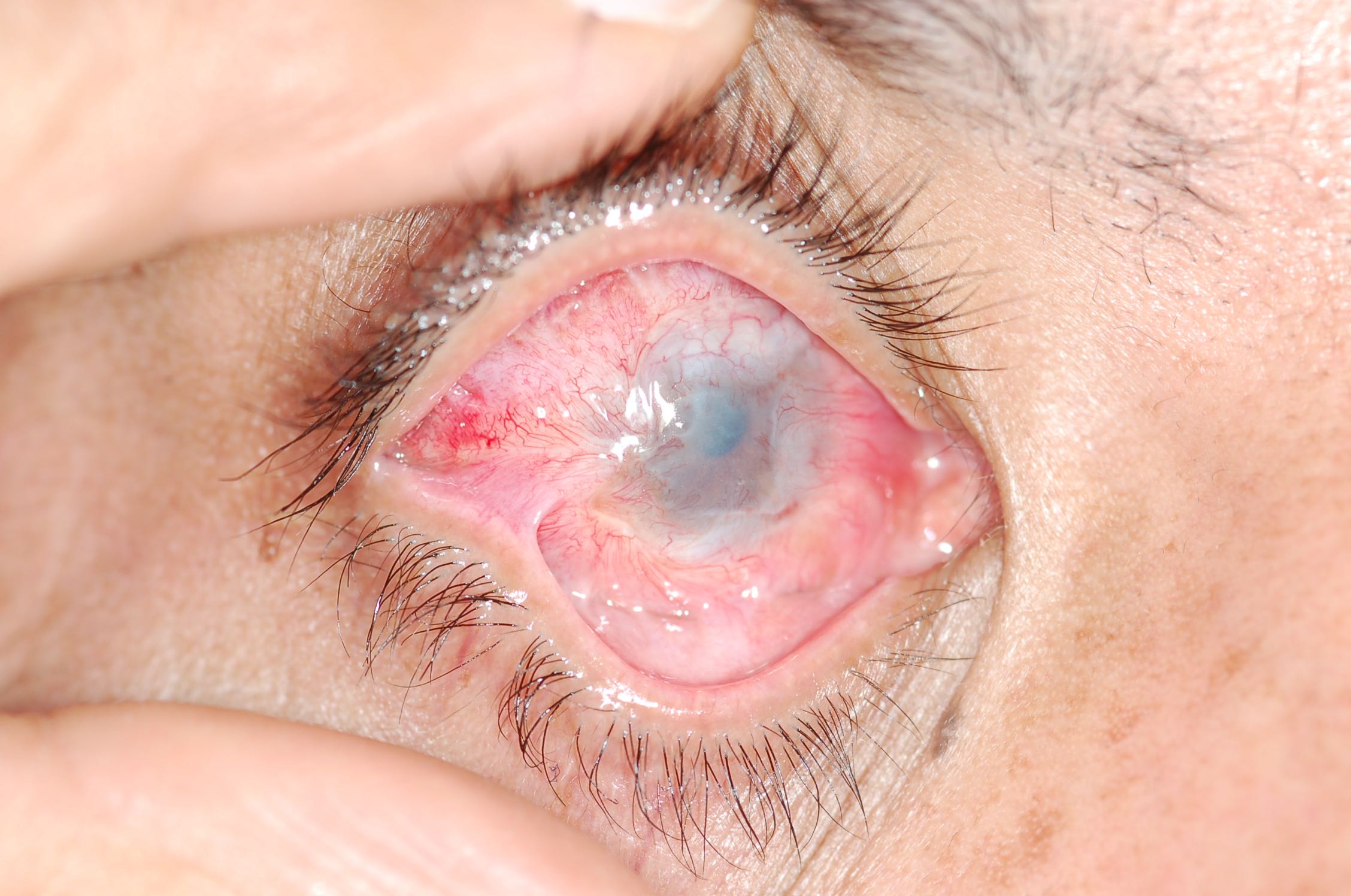
The most common findings represent structural changes to the tissue directly affected, for example, the eye, oral mucosa, skin, esophagus, and lower intestinal system, especially the stomach and pylorus, respiratory system, among others. In children, ingestion is generally the most worrisome event, because of changes, both short-term and long-term, often leading to extensive tissue death. Eye exposure, either acid or alkali, represents a significant acute injury. Copious irrigation is necessary, and measuring pH is appropriate, although rarely informative.
Evaluation
Direct examination of external exposure sites is mandatory, and if there is ingestion, endoscopic evaluation is necessary. In the instance of hydrofluoric (HF) acid exposure (see treatment below), monitoring of serum calcium and magnesium levels is critical to prevent chelation with the fluoride ion and cytotoxicity. With most other topical exposures, observation and serial monitoring of changes are sufficient.[8][9]
Any gastrointestinal (GI) exposure must be seen by an experienced endoscopist who may need to perform serial evaluations to document healing. Likewise, eye injuries must be examined by an experienced ophthalmologist who will follow up with the patient sequentially and guide additional therapy.
With ingestions, especially when concerned about systemic absorption, laboratory evaluation (complete blood count [CBC], platelets, electrolytes, calcium, magnesium, arterial/venous blood gas, liver and kidney studies, lactic acid level, and, occasionally, coagulation studies) may be indicated. Radiographic studies, especially including an upright chest film, may help to determine if there is the presence of free air, which is suggestive of a perforation. Non-contrast CT may be used if there is concern about mediastinal free air, resulting from a perforation after exposure. Previously, a radio-opaque contrast was used, but this should be avoided in suspected perforation.
Treatment / Management
Copious irrigation of affected external areas is mandated. Endoscopic examination best explores internal injuries after ingestion. If there is concern about ingestion of disc or other flat batteries, radiographic assessment is mandated. It would be unusual that CT scanning would be needed, and MRI studies are interdicted. Ultrasonography in experienced hands may provide answers as to location as well.[10][4][11]
It is not appropriate to introduce emetic agents or "neutralizing" agents into the treatment regimen after ingestion. There is high concern about aspiration, increased tissue damage with retching, and a strong possibility of exacerbating a bad situation. There is no current recommendation of systemic medications such as steroids, antibiotics, or prophylactic renal/hepatic therapies.
HF acid, among all the exposures mentioned above, can be treated with copious irrigation and application of a paste (commercially available and often supplied in an industrial setting where HF may be used commonly or made in the emergency department with powdered calcium gluconate and surgical lubricants). Some have recommended benzalkonium chloride solution. When applied, the treating clinician should use barrier protection. In some circumstances, intradermal or intraarterial injections of calcium (gluconate strongly preferred) have been used. Relief of pain is a good marker of the efficacy of treatment. Monitoring of calcium and magnesium levels is important. Oral ingestion, often in the context of suicidal behavior, is likely to be fatal and may be treated with lavage. Monitoring of heart rhythms and electrolytes, including calcium and magnesium, is necessary. Lavage may be helpful, especially if calcium salts are used.
Disc batteries have the potential to leak alkali and cause local, generally esophageal, burns. This is typically seen in children and will require endoscopic management and radiographic tracking of location. Early removal is strongly recommended. If the battery has passed the pylorus, watchful waiting, and inspection of stool for passage are appropriate.
Differential Diagnosis
- Vesicants, Mustard-Hd, Hn1-3
- Magnesium and thermite poisoning
- Ocular burns and chemical injuries
Prognosis
The prognosis depends on the type of chemical and extent of the injury. Most small lesions heal well, but larger wounds often do not heal and can develop into scars. Hydrofluoric acid burns have typically been associated with loss of digits.
Chemical injuries to the eye are the most serious, resulting in severe scarring and permanent loss of vision.
Complications
The most common complications are pain and scarring. Vision loss occurs when the eye is injured. Most patients require multiple doctor visits, and many patients require skin grafts to alleviate the scars.
Postoperative and Rehabilitation Care
Except for first-degree burns, all other burns require some type of follow-up. Skin burns need to be evaluated every 2-4 days until there are signs of healing. Patients with eye burns need to be seen in 24 hours.
For those who suffer a burn to the esophagus, endoscopy has to be repeated in 14-21 days to ensure that there is no stricture formation.
Consultations
Besides a general surgeon or a burn specialist, other consultants involved in the care of these patients include an ophthalmologist, ENT surgeon, gastroenterologist and a pediatrician.
Deterrence and Patient Education
To avoid chemical injury in children, parents should keep all dangerous chemicals out of reach of the children.
Individuals who have attempted suicide with chemicals need a psychiatric referral.
Pearls and Other Issues
Chemical burns have the potential to impair short and long-term health and, especially when the eye or esophagus are involved, severely alter the individual's well-being. The clinician must be vigilant to monitor even minor appearing burns, especially with HF acid, as what initially appears to be minor may have serious side effects.
Enhancing Healthcare Team Outcomes
Because burns can occur on almost any part of the body, specific guidelines in the management of each organ system are lacking. However, there is expert evidence on managing the patient as a whole. However, there still remain several gaps in the early management of chemical burns. What solution to rinse the skin or the eye and when to debride are two issues that continue to be debated. But there is no debate that the eye should be rinsed thoroughly, and the patient must be seen by the ophthalmologist. Because burns can affect all organ systems an interprofessional approach with interaction is necessary to avoid the high morbidity of the disorder.
Since most burn patients are managed in a burn unit, the role of the nurse is vital. Often these professionals are the first to identify burn-related complications like infections, melena, difficulty swallowing, eschar formation, and declining urine output. The pharmacist should be closely involved when burns are caused by medications like podophyllotoxin, formic acid, or topical salicylic acid. Knowledge in managing topical burns, especially in children can help prevent disability.[12][13] (level 3)
Outcomes
The outcomes following a chemical burn depend on the chemical, extent of the burn, comorbidity of the patient, and time to intervention. Some chemicals are more harmful than others, but chemical burns to the eye are always serious. Because chemical burns can cause poor cosmesis and functional disability, a team approach to management is vital.[14] (Level 5)