[1]
Sankri-Tarbichi AG. Obstructive sleep apnea-hypopnea syndrome: Etiology and diagnosis. Avicenna journal of medicine. 2012 Jan:2(1):3-8. doi: 10.4103/2231-0770.94803. Epub
[PubMed PMID: 23210013]
[2]
Mehrtash M, Bakker JP, Ayas N. Predictors of Continuous Positive Airway Pressure Adherence in Patients with Obstructive Sleep Apnea. Lung. 2019 Apr:197(2):115-121. doi: 10.1007/s00408-018-00193-1. Epub 2019 Jan 7
[PubMed PMID: 30617618]
[3]
Esteller E, Carrasco M, Díaz-Herrera MÁ, Vila J, Sampol G, Juvanteny J, Sieira R, Farré A, Vilaseca I. Clinical Practice Guideline recommendations on examination of the upper airway for adults with suspected obstructive sleep apnoea-hypopnoea syndrome. Acta otorrinolaringologica espanola. 2019 Nov-Dec:70(6):364-372. doi: 10.1016/j.otorri.2018.06.008. Epub 2019 Jan 5
[PubMed PMID: 30616837]
Level 1 (high-level) evidence
[4]
Carneiro-Barrera A, Díaz-Román A, Guillén-Riquelme A, Buela-Casal G. Weight loss and lifestyle interventions for obstructive sleep apnoea in adults: Systematic review and meta-analysis. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2019 May:20(5):750-762. doi: 10.1111/obr.12824. Epub 2019 Jan 4
[PubMed PMID: 30609450]
Level 1 (high-level) evidence
[5]
Yeghiazarians Y, Jneid H, Tietjens JR, Redline S, Brown DL, El-Sherif N, Mehra R, Bozkurt B, Ndumele CE, Somers VK. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation. 2021 Jul 20:144(3):e56-e67. doi: 10.1161/CIR.0000000000000988. Epub 2021 Jun 21
[PubMed PMID: 34148375]
[9]
Schwab RJ, Gupta KB, Gefter WB, Metzger LJ, Hoffman EA, Pack AI. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. American journal of respiratory and critical care medicine. 1995 Nov:152(5 Pt 1):1673-89
[PubMed PMID: 7582313]
[10]
Isono S, Remmers JE, Tanaka A, Sho Y, Sato J, Nishino T. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. Journal of applied physiology (Bethesda, Md. : 1985). 1997 Apr:82(4):1319-26
[PubMed PMID: 9104871]
[11]
Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep medicine. 2010 May:11(5):441-6. doi: 10.1016/j.sleep.2009.10.005. Epub 2010 Apr 1
[PubMed PMID: 20362502]
[12]
Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. American journal of respiratory and critical care medicine. 1998 Jan:157(1):144-8
[PubMed PMID: 9445292]
[13]
Dominguez JE, Habib AS. Obstructive sleep apnea in pregnant women. International anesthesiology clinics. 2022 Apr 1:60(2):59-65. doi: 10.1097/AIA.0000000000000360. Epub
[PubMed PMID: 35261345]
[14]
Reutrakul S, Mokhlesi B. Obstructive Sleep Apnea and Diabetes: A State of the Art Review. Chest. 2017 Nov:152(5):1070-1086. doi: 10.1016/j.chest.2017.05.009. Epub 2017 May 17
[PubMed PMID: 28527878]
[15]
Akset M, Poppe KG, Kleynen P, Bold I, Bruyneel M. Endocrine disorders in obstructive sleep apnoea syndrome: A bidirectional relationship. Clinical endocrinology. 2023 Jan:98(1):3-13. doi: 10.1111/cen.14685. Epub 2022 Feb 19
[PubMed PMID: 35182448]
[17]
Sankari A, Bascom A, Oomman S, Badr MS. Sleep disordered breathing in chronic spinal cord injury. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2014 Jan 15:10(1):65-72. doi: 10.5664/jcsm.3362. Epub 2014 Jan 15
[PubMed PMID: 24426822]
[18]
Kim SJ, Cho SY, Jin DK. Prader-Willi syndrome: an update on obesity and endocrine problems. Annals of pediatric endocrinology & metabolism. 2021 Dec:26(4):227-236. doi: 10.6065/apem.2142164.082. Epub 2021 Dec 31
[PubMed PMID: 34991300]
[19]
Hyzer JM, Milczuk HA, Macarthur CJ, King EF, Quintanilla-Dieck L, Lam DJ. Drug-Induced Sleep Endoscopy Findings in Children With Obstructive Sleep Apnea With vs Without Obesity or Down Syndrome. JAMA otolaryngology-- head & neck surgery. 2021 Feb 1:147(2):175-181. doi: 10.1001/jamaoto.2020.4548. Epub
[PubMed PMID: 33270102]
[20]
Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. American journal of respiratory and critical care medicine. 1999 Oct:160(4):1101-6
[PubMed PMID: 10508793]
[21]
Moula AI, Parrini I, Tetta C, Lucà F, Parise G, Rao CM, Mauro E, Parise O, Matteucci F, Gulizia MM, La Meir M, Gelsomino S. Obstructive Sleep Apnea and Atrial Fibrillation. Journal of clinical medicine. 2022 Feb 25:11(5):. doi: 10.3390/jcm11051242. Epub 2022 Feb 25
[PubMed PMID: 35268335]
[22]
Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. The New England journal of medicine. 1993 Apr 29:328(17):1230-5
[PubMed PMID: 8464434]
[23]
Malhotra A, Ayappa I, Ayas N, Collop N, Kirsch D, Mcardle N, Mehra R, Pack AI, Punjabi N, White DP, Gottlieb DJ. Metrics of sleep apnea severity: beyond the apnea-hypopnea index. Sleep. 2021 Jul 9:44(7):. doi: 10.1093/sleep/zsab030. Epub
[PubMed PMID: 33693939]
[24]
Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, Nunez CM, Patel SR, Penzel T, Pépin JL, Peppard PE, Sinha S, Tufik S, Valentine K, Malhotra A. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. The Lancet. Respiratory medicine. 2019 Aug:7(8):687-698. doi: 10.1016/S2213-2600(19)30198-5. Epub 2019 Jul 9
[PubMed PMID: 31300334]
[25]
Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. American journal of epidemiology. 2013 May 1:177(9):1006-14. doi: 10.1093/aje/kws342. Epub 2013 Apr 14
[PubMed PMID: 23589584]
[26]
Gottlieb DJ, Punjabi NM. Diagnosis and Management of Obstructive Sleep Apnea: A Review. JAMA. 2020 Apr 14:323(14):1389-1400. doi: 10.1001/jama.2020.3514. Epub
[PubMed PMID: 32286648]
[27]
Garvey JF, Pengo MF, Drakatos P, Kent BD. Epidemiological aspects of obstructive sleep apnea. Journal of thoracic disease. 2015 May:7(5):920-9. doi: 10.3978/j.issn.2072-1439.2015.04.52. Epub
[PubMed PMID: 26101650]
Level 2 (mid-level) evidence
[28]
Morrell MJ, Arabi Y, Zahn B, Badr MS. Progressive retropalatal narrowing preceding obstructive apnea. American journal of respiratory and critical care medicine. 1998 Dec:158(6):1974-81
[PubMed PMID: 9847295]
[29]
Sankri-Tarbichi AG, Rowley JA, Badr MS. Expiratory pharyngeal narrowing during central hypocapnic hypopnea. American journal of respiratory and critical care medicine. 2009 Feb 15:179(4):313-9. doi: 10.1164/rccm.200805-741OC. Epub 2008 Nov 21
[PubMed PMID: 19201929]
[30]
Smith PL, Wise RA, Gold AR, Schwartz AR, Permutt S. Upper airway pressure-flow relationships in obstructive sleep apnea. Journal of applied physiology (Bethesda, Md. : 1985). 1988 Feb:64(2):789-95
[PubMed PMID: 3372436]
[31]
Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991 Dec:14(6):540-5
[PubMed PMID: 1798888]
[32]
Learmonth YC, Dlugonski D, Pilutti LA, Sandroff BM, Klaren R, Motl RW. Psychometric properties of the Fatigue Severity Scale and the Modified Fatigue Impact Scale. Journal of the neurological sciences. 2013 Aug 15:331(1-2):102-7. doi: 10.1016/j.jns.2013.05.023. Epub 2013 Jun 20
[PubMed PMID: 23791482]
[33]
Russell MB, Kristiansen HA, Kværner KJ. Headache in sleep apnea syndrome: epidemiology and pathophysiology. Cephalalgia : an international journal of headache. 2014 Sep:34(10):752-5. doi: 10.1177/0333102414538551. Epub 2014 Jun 13
[PubMed PMID: 24928423]
[34]
Cho YW, Kim KT, Moon HJ, Korostyshevskiy VR, Motamedi GK, Yang KI. Comorbid Insomnia With Obstructive Sleep Apnea: Clinical Characteristics and Risk Factors. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2018 Mar 15:14(3):409-417. doi: 10.5664/jcsm.6988. Epub 2018 Mar 15
[PubMed PMID: 29458695]
[35]
Maeda T, Fukunaga K, Nagata H, Haraguchi M, Kikuchi E, Miyajima A, Yamasawa W, Shirahama R, Narita M, Betsuyaku T, Asano K, Oya M. Obstructive sleep apnea syndrome should be considered as a cause of nocturia in younger patients without other voiding symptoms. Canadian Urological Association journal = Journal de l'Association des urologues du Canada. 2016 Jul-Aug:10(7-8):E241-E245. doi: 10.5489/cuaj.3508. Epub 2016 Jul 12
[PubMed PMID: 28255415]
[36]
Subramanian S, Guntupalli B, Murugan T, Bopparaju S, Chanamolu S, Casturi L, Surani S. Gender and ethnic differences in prevalence of self-reported insomnia among patients with obstructive sleep apnea. Sleep & breathing = Schlaf & Atmung. 2011 Dec:15(4):711-5. doi: 10.1007/s11325-010-0426-4. Epub 2010 Oct 16
[PubMed PMID: 20953842]
[37]
Nagappa M, Liao P, Wong J, Auckley D, Ramachandran SK, Memtsoudis S, Mokhlesi B, Chung F. Validation of the STOP-Bang Questionnaire as a Screening Tool for Obstructive Sleep Apnea among Different Populations: A Systematic Review and Meta-Analysis. PloS one. 2015:10(12):e0143697. doi: 10.1371/journal.pone.0143697. Epub 2015 Dec 14
[PubMed PMID: 26658438]
Level 1 (high-level) evidence
[38]
Schellenberg JB, Maislin G, Schwab RJ. Physical findings and the risk for obstructive sleep apnea. The importance of oropharyngeal structures. American journal of respiratory and critical care medicine. 2000 Aug:162(2 Pt 1):740-8
[PubMed PMID: 10934114]
[39]
Jonas DE, Amick HR, Feltner C, Weber RP, Arvanitis M, Stine A, Lux L, Harris RP. Screening for Obstructive Sleep Apnea in Adults: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2017 Jan 24:317(4):415-433. doi: 10.1001/jama.2016.19635. Epub
[PubMed PMID: 28118460]
Level 1 (high-level) evidence
[40]
Colvin LJ, Collop NA. Commercial Motor Vehicle Driver Obstructive Sleep Apnea Screening and Treatment in the United States: An Update and Recommendation Overview. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2016 Jan:12(1):113-25. doi: 10.5664/jcsm.5408. Epub
[PubMed PMID: 26094916]
Level 3 (low-level) evidence
[41]
Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004 Apr 28:291(16):2013-6
[PubMed PMID: 15113821]
[42]
Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, Redline S, Strohl KP, Davidson Ward SL, Tangredi MM, American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2012 Oct 15:8(5):597-619. doi: 10.5664/jcsm.2172. Epub 2012 Oct 15
[PubMed PMID: 23066376]
[43]
Berry RB, Brooks R, Gamaldo C, Harding SM, Lloyd RM, Quan SF, Troester MT, Vaughn BV. AASM Scoring Manual Updates for 2017 (Version 2.4). Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2017 May 15:13(5):665-666. doi: 10.5664/jcsm.6576. Epub 2017 May 15
[PubMed PMID: 28416048]
[44]
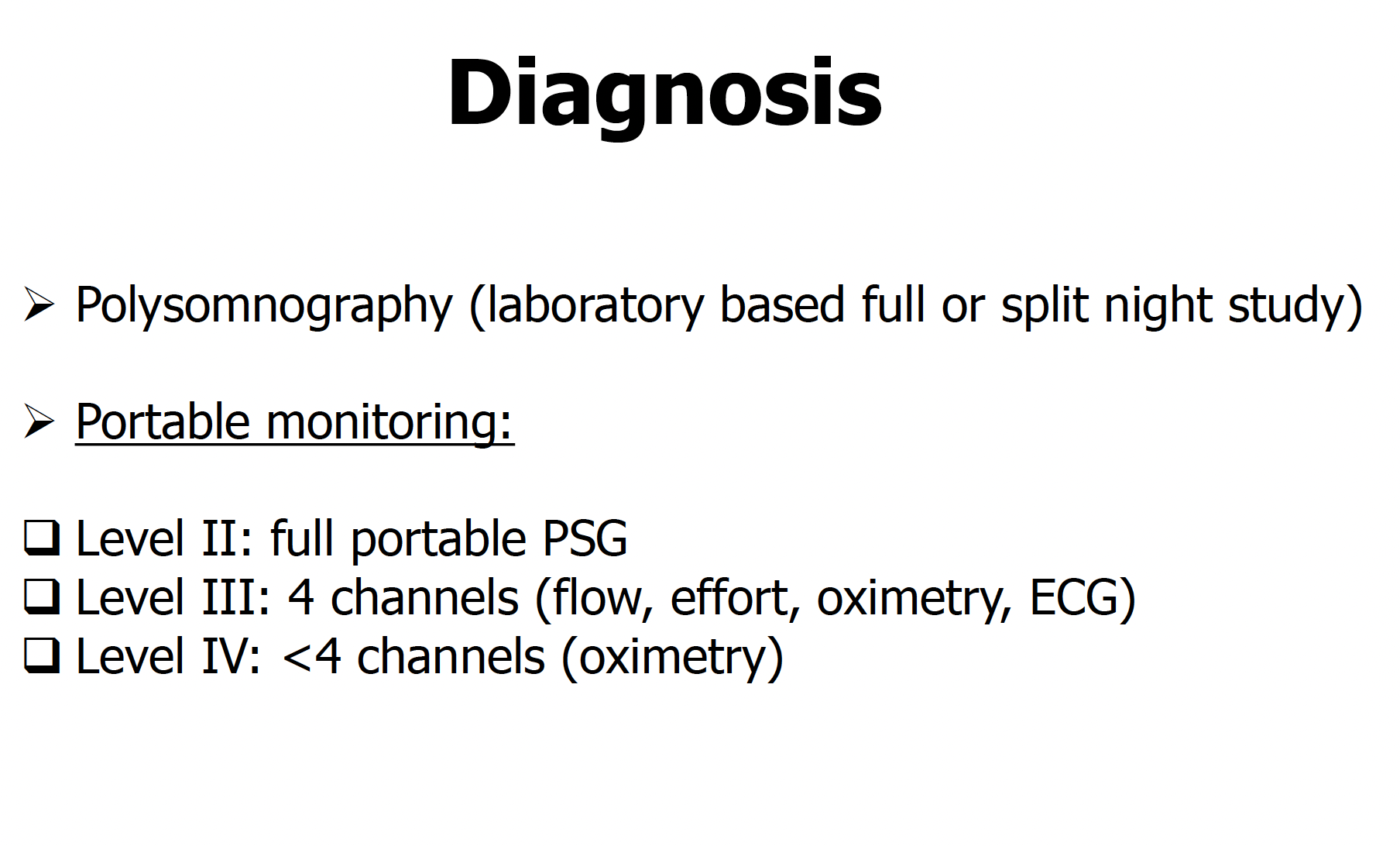
Collop NA, Anderson WM, Boehlecke B, Claman D, Goldberg R, Gottlieb DJ, Hudgel D, Sateia M, Schwab R, Portable Monitoring Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2007 Dec 15:3(7):737-47
[PubMed PMID: 18198809]
[45]
Light MP, Casimire TN, Chua C, Koushyk V, Burschtin OE, Ayappa I, Rapoport DM. Addition of frontal EEG to adult home sleep apnea testing: does a more accurate determination of sleep time make a difference? Sleep & breathing = Schlaf & Atmung. 2018 Dec:22(4):1179-1188. doi: 10.1007/s11325-018-1735-2. Epub 2018 Oct 11
[PubMed PMID: 30311183]
[46]
Hedner J, White DP, Malhotra A, Herscovici S, Pittman SD, Zou D, Grote L, Pillar G. Sleep staging based on autonomic signals: a multi-center validation study. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2011 Jun 15:7(3):301-6. doi: 10.5664/JCSM.1078. Epub
[PubMed PMID: 21677901]
Level 1 (high-level) evidence
[47]
Chowdhuri S, Quan SF, Almeida F, Ayappa I, Batool-Anwar S, Budhiraja R, Cruse PE, Drager LF, Griss B, Marshall N, Patel SR, Patil S, Knight SL, Rowley JA, Slyman A, ATS Ad Hoc Committee on Mild Obstructive Sleep Apnea. An Official American Thoracic Society Research Statement: Impact of Mild Obstructive Sleep Apnea in Adults. American journal of respiratory and critical care medicine. 2016 May 1:193(9):e37-54. doi: 10.1164/rccm.201602-0361ST. Epub
[PubMed PMID: 27128710]
[48]
Yasir M, Pervaiz A, Sankari A. Cardiovascular Outcomes in Sleep-Disordered Breathing: Are We Under-estimating? Frontiers in neurology. 2022:13():801167. doi: 10.3389/fneur.2022.801167. Epub 2022 Mar 15
[PubMed PMID: 35370882]
[49]
Sankari A, Ravelo LA, Maresh S, Aljundi N, Alsabri B, Fawaz S, Hamdon M, Al-Kubaisi G, Hagen E, Badr MS, Peppard P. Longitudinal effect of nocturnal R-R intervals changes on cardiovascular outcome in a community-based cohort. BMJ open. 2019 Jul 17:9(7):e030559. doi: 10.1136/bmjopen-2019-030559. Epub 2019 Jul 17
[PubMed PMID: 31315880]
[50]
Azarbarzin A, Sands SA, Stone KL, Taranto-Montemurro L, Messineo L, Terrill PI, Ancoli-Israel S, Ensrud K, Purcell S, White DP, Redline S, Wellman A. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. European heart journal. 2019 Apr 7:40(14):1149-1157. doi: 10.1093/eurheartj/ehy624. Epub
[PubMed PMID: 30376054]
[51]
Oldenburg O, Wellmann B, Buchholz A, Bitter T, Fox H, Thiem U, Horstkotte D, Wegscheider K. Nocturnal hypoxaemia is associated with increased mortality in stable heart failure patients. European heart journal. 2016 Jun 1:37(21):1695-703. doi: 10.1093/eurheartj/ehv624. Epub 2015 Nov 26
[PubMed PMID: 26612581]
[52]
Butler MP, Emch JT, Rueschman M, Sands SA, Shea SA, Wellman A, Redline S. Apnea-Hypopnea Event Duration Predicts Mortality in Men and Women in the Sleep Heart Health Study. American journal of respiratory and critical care medicine. 2019 Apr 1:199(7):903-912. doi: 10.1164/rccm.201804-0758OC. Epub
[PubMed PMID: 30336691]
[53]
Shahrbabaki SS, Linz D, Hartmann S, Redline S, Baumert M. Sleep arousal burden is associated with long-term all-cause and cardiovascular mortality in 8001 community-dwelling older men and women. European heart journal. 2021 Jun 1:42(21):2088-2099. doi: 10.1093/eurheartj/ehab151. Epub
[PubMed PMID: 33876221]
[54]
Khalyfa A, Zhang C, Khalyfa AA, Foster GE, Beaudin AE, Andrade J, Hanly PJ, Poulin MJ, Gozal D. Effect on Intermittent Hypoxia on Plasma Exosomal Micro RNA Signature and Endothelial Function in Healthy Adults. Sleep. 2016 Dec 1:39(12):2077-2090. doi: 10.5665/sleep.6302. Epub 2016 Dec 1
[PubMed PMID: 27634792]
[55]
Li K, Wei P, Qin Y, Wei Y. MicroRNA expression profiling and bioinformatics analysis of dysregulated microRNAs in obstructive sleep apnea patients. Medicine. 2017 Aug:96(34):e7917. doi: 10.1097/MD.0000000000007917. Epub
[PubMed PMID: 28834917]
Level 2 (mid-level) evidence
[56]
da Silva Paulitsch F, Zhang L. Continuous positive airway pressure for adults with obstructive sleep apnea and cardiovascular disease: a meta-analysis of randomized trials. Sleep medicine. 2019 Feb:54():28-34. doi: 10.1016/j.sleep.2018.09.030. Epub 2018 Oct 30
[PubMed PMID: 30529774]
Level 1 (high-level) evidence
[57]
Ng SSS, Tam WWS, Lee RWW, Chan TO, Yiu K, Yuen BTY, Wong KT, Woo J, Ma RCW, Chan KKP, Ko FWS, Cistulli PA, Hui DS. Effect of Weight Loss and Continuous Positive Airway Pressure on Obstructive Sleep Apnea and Metabolic Profile Stratified by Craniofacial Phenotype: A Randomized Clinical Trial. American journal of respiratory and critical care medicine. 2022 Mar 15:205(6):711-720. doi: 10.1164/rccm.202106-1401OC. Epub
[PubMed PMID: 34936531]
Level 1 (high-level) evidence
[58]
Wong AM, Barnes HN, Joosten SA, Landry SA, Dabscheck E, Mansfield DR, Dharmage SC, Senaratna CV, Edwards BA, Hamilton GS. The effect of surgical weight loss on obstructive sleep apnoea: A systematic review and meta-analysis. Sleep medicine reviews. 2018 Dec:42():85-99. doi: 10.1016/j.smrv.2018.06.001. Epub 2018 Jun 19
[PubMed PMID: 30001806]
Level 1 (high-level) evidence
[59]
Al Lawati NM, Patel SR, Ayas NT. Epidemiology, risk factors, and consequences of obstructive sleep apnea and short sleep duration. Progress in cardiovascular diseases. 2009 Jan-Feb:51(4):285-93. doi: 10.1016/j.pcad.2008.08.001. Epub
[PubMed PMID: 19110130]
[60]
Cerritelli L, Caranti A, Migliorelli A, Bianchi G, Stringa LM, Bonsembiante A, Cammaroto G, Pelucchi S, Vicini C. Sleep position and obstructive sleep apnea (OSA): Do we know how we sleep? A new explorative sleeping questionnaire. Sleep & breathing = Schlaf & Atmung. 2022 Dec:26(4):1973-1981. doi: 10.1007/s11325-022-02576-4. Epub 2022 Feb 7
[PubMed PMID: 35129756]
[61]
Jo JH, Kim SH, Jang JH, Park JW, Chung JW. Comparison of polysomnographic and cephalometric parameters based on positional and rapid eye movement sleep dependency in obstructive sleep apnea. Scientific reports. 2022 Jun 14:12(1):9828. doi: 10.1038/s41598-022-13850-6. Epub 2022 Jun 14
[PubMed PMID: 35701572]
[62]
Ravesloot MJ, de Vries N. Reliable calculation of the efficacy of non-surgical and surgical treatment of obstructive sleep apnea revisited. Sleep. 2011 Jan 1:34(1):105-10
[PubMed PMID: 21203364]
[63]
Xanthopoulos MS, Kim JY, Blechner M, Chang MY, Menello MK, Brown C, Matthews E, Weaver TE, Shults J, Marcus CL. Self-Efficacy and Short-Term Adherence to Continuous Positive Airway Pressure Treatment in Children. Sleep. 2017 Jul 1:40(7):. doi: 10.1093/sleep/zsx096. Epub
[PubMed PMID: 28541508]
[64]
Schwab RJ, Badr SM, Epstein LJ, Gay PC, Gozal D, Kohler M, Lévy P, Malhotra A, Phillips BA, Rosen IM, Strohl KP, Strollo PJ, Weaver EM, Weaver TE, ATS Subcommittee on CPAP Adherence Tracking Systems. An official American Thoracic Society statement: continuous positive airway pressure adherence tracking systems. The optimal monitoring strategies and outcome measures in adults. American journal of respiratory and critical care medicine. 2013 Sep 1:188(5):613-20. doi: 10.1164/rccm.201307-1282ST. Epub
[PubMed PMID: 23992588]
[65]
Fox N, Hirsch-Allen AJ, Goodfellow E, Wenner J, Fleetham J, Ryan CF, Kwiatkowska M, Ayas NT. The impact of a telemedicine monitoring system on positive airway pressure adherence in patients with obstructive sleep apnea: a randomized controlled trial. Sleep. 2012 Apr 1:35(4):477-81. doi: 10.5665/sleep.1728. Epub 2012 Apr 1
[PubMed PMID: 22467985]
Level 1 (high-level) evidence
[66]
Bakker JP, Weaver TE, Parthasarathy S, Aloia MS. Adherence to CPAP: What Should We Be Aiming For, and How Can We Get There? Chest. 2019 Jun:155(6):1272-1287. doi: 10.1016/j.chest.2019.01.012. Epub 2019 Jan 23
[PubMed PMID: 30684472]
[67]
Khan NNS, Todem D, Bottu S, Badr MS, Olomu A. Impact of patient and family engagement in improving continuous positive airway pressure adherence in patients with obstructive sleep apnea: a randomized controlled trial. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2022 Jan 1:18(1):181-191. doi: 10.5664/jcsm.9534. Epub
[PubMed PMID: 34270409]
Level 1 (high-level) evidence
[68]
Thong BKS, Loh GXY, Lim JJ, Lee CJL, Ting SN, Li HP, Li QY. Telehealth Technology Application in Enhancing Continuous Positive Airway Pressure Adherence in Obstructive Sleep Apnea Patients: A Review of Current Evidence. Frontiers in medicine. 2022:9():877765. doi: 10.3389/fmed.2022.877765. Epub 2022 May 3
[PubMed PMID: 35592853]
[69]
Sánchez-de-la-Torre M, Sánchez-de-la-Torre A, Bertran S, Abad J, Duran-Cantolla J, Cabriada V, Mediano O, Masdeu MJ, Alonso ML, Masa JF, Barceló A, de la Peña M, Mayos M, Coloma R, Montserrat JM, Chiner E, Perelló S, Rubinós G, Mínguez O, Pascual L, Cortijo A, Martínez D, Aldomà A, Dalmases M, McEvoy RD, Barbé F, Spanish Sleep Network. Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): a randomised controlled trial. The Lancet. Respiratory medicine. 2020 Apr:8(4):359-367. doi: 10.1016/S2213-2600(19)30271-1. Epub 2019 Dec 12
[PubMed PMID: 31839558]
Level 1 (high-level) evidence
[70]
Lisan Q, Van Sloten T, Marques Vidal P, Haba Rubio J, Heinzer R, Empana JP. Association of Positive Airway Pressure Prescription With Mortality in Patients With Obesity and Severe Obstructive Sleep Apnea: The Sleep Heart Health Study. JAMA otolaryngology-- head & neck surgery. 2019 Jun 1:145(6):509-515. doi: 10.1001/jamaoto.2019.0281. Epub
[PubMed PMID: 30973594]
[71]
Azarbarzin A, Zinchuk A, Wellman A, Labarca G, Vena D, Gell L, Messineo L, White DP, Gottlieb DJ, Redline S, Peker Y, Sands SA. Cardiovascular Benefit of Continuous Positive Airway Pressure in Adults with Coronary Artery Disease and Obstructive Sleep Apnea without Excessive Sleepiness. American journal of respiratory and critical care medicine. 2022 Sep 15:206(6):767-774. doi: 10.1164/rccm.202111-2608OC. Epub
[PubMed PMID: 35579605]
[72]
Phillips CL, Grunstein RR, Darendeliler MA, Mihailidou AS, Srinivasan VK, Yee BJ, Marks GB, Cistulli PA. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. American journal of respiratory and critical care medicine. 2013 Apr 15:187(8):879-87. doi: 10.1164/rccm.201212-2223OC. Epub
[PubMed PMID: 23413266]
Level 1 (high-level) evidence
[73]
Uniken Venema JAM, Doff MHJ, Joffe-Sokolova D, Wijkstra PJ, van der Hoeven JH, Stegenga B, Hoekema A. Long-term obstructive sleep apnea therapy: a 10-year follow-up of mandibular advancement device and continuous positive airway pressure. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2020 Mar 15:16(3):353-359. doi: 10.5664/jcsm.8204. Epub 2020 Jan 14
[PubMed PMID: 31992403]
[74]
Ramar K, Dort LC, Katz SG, Lettieri CJ, Harrod CG, Thomas SM, Chervin RD. Clinical Practice Guideline for the Treatment of Obstructive Sleep Apnea and Snoring with Oral Appliance Therapy: An Update for 2015. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2015 Jul 15:11(7):773-827. doi: 10.5664/jcsm.4858. Epub 2015 Jul 15
[PubMed PMID: 26094920]
Level 1 (high-level) evidence
[75]
Maniaci A, Di Luca M, Lechien JR, Iannella G, Grillo C, Grillo CM, Merlino F, Calvo-Henriquez C, De Vito A, Magliulo G, Pace A, Vicini C, Cocuzza S, Bannò V, Pollicina I, Stilo G, Bianchi A, La Mantia I. Lateral pharyngoplasty vs. traditional uvulopalatopharyngoplasty for patients with OSA: systematic review and meta-analysis. Sleep & breathing = Schlaf & Atmung. 2022 Dec:26(4):1539-1550. doi: 10.1007/s11325-021-02520-y. Epub 2022 Jan 3
[PubMed PMID: 34978022]
Level 1 (high-level) evidence
[76]
He M, Yin G, Zhan S, Xu J, Cao X, Li J, Ye J. Long-term Efficacy of Uvulopalatopharyngoplasty among Adult Patients with Obstructive Sleep Apnea: A Systematic Review and Meta-analysis. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2019 Sep:161(3):401-411. doi: 10.1177/0194599819840356. Epub 2019 Jun 11
[PubMed PMID: 31184261]
Level 1 (high-level) evidence
[77]
Martin MJ, Khanna A, Srinivasan D, Sovani MP. Patient-reported outcome measures following maxillomandibular advancement surgery in patients with obstructive sleep apnoea syndrome. The British journal of oral & maxillofacial surgery. 2022 Sep:60(7):963-968. doi: 10.1016/j.bjoms.2022.03.006. Epub 2022 Mar 22
[PubMed PMID: 35667944]
[78]
Zhou N, Ho JTF, de Vries N, Bosschieter PFN, Ravesloot MJL, de Lange J. Evaluation of drug-induced sleep endoscopy as a tool for selecting patients with obstructive sleep apnea for maxillomandibular advancement. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2022 Apr 1:18(4):1073-1081. doi: 10.5664/jcsm.9802. Epub
[PubMed PMID: 34877928]
[79]
Huang Z, Bosschieter PFN, Aarab G, van Selms MKA, Vanhommerig JW, Hilgevoord AAJ, Lobbezoo F, de Vries N. Predicting upper airway collapse sites found in drug-induced sleep endoscopy from clinical data and snoring sounds in patients with obstructive sleep apnea: a prospective clinical study. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2022 Sep 1:18(9):2119-2131. doi: 10.5664/jcsm.9998. Epub
[PubMed PMID: 35459443]
[80]
Lewis R, Pételle B, Campbell MC, MacKay S, Palme C, Raux G, Sommer JU, Maurer JT. Implantation of the nyxoah bilateral hypoglossal nerve stimulator for obstructive sleep apnea. Laryngoscope investigative otolaryngology. 2019 Dec:4(6):703-707. doi: 10.1002/lio2.312. Epub 2019 Nov 22
[PubMed PMID: 31890891]
[81]
Kompelli AR, Ni JS, Nguyen SA, Lentsch EJ, Neskey DM, Meyer TA. The outcomes of hypoglossal nerve stimulation in the management of OSA: A systematic review and meta-analysis. World journal of otorhinolaryngology - head and neck surgery. 2019 Mar:5(1):41-48. doi: 10.1016/j.wjorl.2018.04.006. Epub 2018 Sep 25
[PubMed PMID: 30775701]
Level 1 (high-level) evidence
[82]
Strollo PJ Jr, Soose RJ, Maurer JT, de Vries N, Cornelius J, Froymovich O, Hanson RD, Padhya TA, Steward DL, Gillespie MB, Woodson BT, Van de Heyning PH, Goetting MG, Vanderveken OM, Feldman N, Knaack L, Strohl KP, STAR Trial Group. Upper-airway stimulation for obstructive sleep apnea. The New England journal of medicine. 2014 Jan 9:370(2):139-49. doi: 10.1056/NEJMoa1308659. Epub
[PubMed PMID: 24401051]
[83]
Bellamkonda N, Shiba T, Mendelsohn AH. Adverse Events in Hypoglossal Nerve Stimulator Implantation: 5-Year Analysis of the FDA MAUDE Database. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2021 Feb:164(2):443-447. doi: 10.1177/0194599820960069. Epub 2020 Sep 22
[PubMed PMID: 32957866]
[84]
Guralnick AS, Balachandran JS, Szutenbach S, Adley K, Emami L, Mohammadi M, Farnan JM, Arora VM, Mokhlesi B. Educational video to improve CPAP use in patients with obstructive sleep apnoea at risk for poor adherence: a randomised controlled trial. Thorax. 2017 Dec:72(12):1132-1139. doi: 10.1136/thoraxjnl-2017-210106. Epub 2017 Jun 30
[PubMed PMID: 28667231]
Level 1 (high-level) evidence
[85]
Marshall NS, Wong KK, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2014 Apr 15:10(4):355-62. doi: 10.5664/jcsm.3600. Epub 2014 Apr 15
[PubMed PMID: 24733978]
[86]
Bock JM, Needham KA, Gregory DA, Ekono MM, Wickwire EM, Somers VK, Lerman A. Continuous Positive Airway Pressure Adherence and Treatment Cost in Patients With Obstructive Sleep Apnea and Cardiovascular Disease. Mayo Clinic proceedings. Innovations, quality & outcomes. 2022 Apr:6(2):166-175. doi: 10.1016/j.mayocpiqo.2022.01.002. Epub 2022 Apr 4
[PubMed PMID: 35399584]
Level 2 (mid-level) evidence
[87]
Alkatib S, Sankri-Tarbichi AG, Badr MS. The impact of obesity on cardiac dysfunction in patients with sleep-disordered breathing. Sleep & breathing = Schlaf & Atmung. 2014 Mar:18(1):137-42. doi: 10.1007/s11325-013-0861-0. Epub 2013 May 15
[PubMed PMID: 23673872]
[88]
Saeed S, Romarheim A, Solheim E, Bjorvatn B, Lehmann S. Cardiovascular remodeling in obstructive sleep apnea: focus on arterial stiffness, left ventricular geometry and atrial fibrillation. Expert review of cardiovascular therapy. 2022 Jun:20(6):455-464. doi: 10.1080/14779072.2022.2081547. Epub 2022 Jun 8
[PubMed PMID: 35673889]
[89]
Tadic M, Gherbesi E, Faggiano A, Sala C, Carugo S, Cuspidi C. The impact of continuous positive airway pressure on cardiac mechanics: Findings from a meta-analysis of echocardiographic studies. Journal of clinical hypertension (Greenwich, Conn.). 2022 Jul:24(7):795-803. doi: 10.1111/jch.14488. Epub 2022 Jun 13
[PubMed PMID: 35695237]
Level 1 (high-level) evidence