Continuing Education Activity
Rhodococcus equi is a bacterium primarily associated with animals, particularly horses and foals. Although this bacterium has been identified as a rare organism, significant case reports of human infections have been reported since 1967. Belonging to the Nocardiaceae family, which also includes pathogenic species such as R fascians, R erythropoiesis, and R rhodochrous, R equi poses a specific risk to individuals with immunosuppressive conditions. R equi is most accurately characterized as a soil organism present in the gastrointestinal tract of numerous herbivores and prevalent in animal dung, manures, soils of grazing fields, and other associated farm environments. The number of organisms isolated from the air increases with warmer temperatures, reaching its peak on dry and windy days.
R equi is a rare contributor to subacute, necrotizing pneumonia, resulting in cavitary pneumonia and lung abscesses. Individuals with weakened immune systems or immunocompromised conditions in which the infection has been described, including those undergoing chemotherapy, having HIV, leukemia, lymphoma, or lung cancer, those with prolonged steroid use, or those receiving monoclonal antibodies or solid organ or stem cell transplants, are susceptible to infections. This activity examines the pathophysiology of this zoonotic infection, its typical presentation in human hosts, and the recommended treatment. The activity highlights the crucial role of an interprofessional healthcare team in assessing the evaluation of R equi infections and caring for patients with this condition to enhance clinical outcomes.
Objectives:
Identify Rhodococcus equi infections by recognizing clinical manifestations and considering epidemiologic exposure to farm animals in immunocompromised patients.
Screen high-risk individuals, especially those with immunosuppressive conditions, for Rhodococcus equi infections through targeted clinical and laboratory assessments.
Select antimicrobial agents, such as extended-spectrum macrolides or fluoroquinolones, based on susceptibility patterns and patient-specific factors for Rhodococcus equi infections.
Collaborate within an interprofessional healthcare team to coordinate follow-up care, monitor treatment response, and address potential complications or relapses in Rhodococcus equi infections.
Introduction
Rhodococcus equi is a bacterium primarily associated with animals, particularly horses and foals, which are the natural hosts. Since 1967, significant cases of human infections have been reported with this rare bacterium, which was first identified by a young man working on immunosuppressant agents in a stockyard.[1] Other Rhodococcus spp, including R fascias, R rhodochrous, and R erythropoiesis, are similarly described as human pathogens. Rhodococcus belongs to the Nocardiaceae family, which also comprises Nocardia, Mycobacterium, Corynebacterium, and Gordonia, exhibiting certain similarities among the group.[2][3]
R equi is most accurately characterized as a soil organism present in the gastrointestinal tract of numerous herbivores and prevalent in animal dung, manures, soils of grazing fields, and other associated farm environments. Although R equi is considered a rare organism, it poses a specific risk to individuals with immunosuppressive conditions. Immunosuppression, especially defects in cell-mediated immunity, significantly contributes to the disease and is present in most reported cases. Individuals with weakened immune systems or immunocompromised conditions in which the infection has been described, including those undergoing chemotherapy, having HIV, leukemia, lymphoma, or lung cancer, those with prolonged steroid use, or those receiving monoclonal antibodies or solid organ or stem cell transplants, are susceptible to infections.[4] R equi is a rare contributor to subacute, necrotizing pneumonia, resulting in cavitary pneumonia and lung abscesses. The condition predominantly causes pulmonary infections, ranging from simple pneumonia to necrotizing pneumonia and lung abscesses.[5]
Etiology
The pathogen, R equi, was formerly known as Corynebacterium equi for several decades, and then it was reclassified as the genus Rhodococcus in 1980. R equi is a gram-positive, obligate aerobic coccobacillus that lacks motility. Although characteristically, R equi produces a red pigment, particularly noticeable in older cultures, younger cultures typically exhibit a pale salmon-pink color on solid media.[6][5][7]
R equi is classified as a "nocardioform" actinomycete and is a partially acid-fast, non-spore–forming, facultative intracellular, and pleomorphic bacterium.[5] The organism resides on the phagosome of the host cell and thereby resists macrophage killing by inhibiting lysosome-phagosome fusion.[5] This resistance results in tissue destruction and neutrophil influx, potentially leading to necrotizing pneumonia.
Vesicle-associated membrane protein (VAPA) facilitates the intramacrophage proliferation of this organism and has been reported in R equi strains causing human infections.[5] Notably, not all disease-causing R equi strains exhibit positivity for VAPA.[5]
The organism is ubiquitous in soil contaminated with herbivore manure. More than 50% of reported cases involve contact with farm animals, particularly horses, exposure to farm dust, and cohabitation with individuals infected with this disease.[8] Airborne dust particles are the primary vehicle for transmitting this infection to humans.[9] Ecological studies on the disease burden in farm animals indicate that the prevalence of R equi pneumonia is associated with the airborne load of virulent R equi.[10]
Although sporadic case reports of infection in immunocompetent individuals have been reported, this disease predominantly affects immunocompromised individuals. According to a systematic review, HIV infection was the primary risk factor in 61.5% of patients affected by this disease.[5] Other comorbid conditions identified as risk factors include malignancies, immunosuppressive therapy such as corticosteroid use, and transplant recipients.[5]
Case reports documenting R equi infections in immunocompetent individuals identify exposure to horses. In a series of 72 cases, 32% were associated with horse or other farm animal exposure, irrespective of immune status.[5]
Epidemiology
Infection with R equi has been reported on every continent except Antarctica. R equi is most accurately characterized as a soil organism present in the gastrointestinal tract of numerous herbivores and prevalent in animal dung, manures, soils of grazing fields, and other associated farm environments. The number of organisms isolated from the air increases with warmer temperatures, reaching its peak on dry and windy days.[11]
R equi is ubiquitous in solid and readily multiples in herbivore manure.[9] Exposure to soil contaminated with herbivore manure, whether through inhalation or ingesting contaminated material, is postulated as the entry route.[12][9] Reports also indicate traumatic inoculation and superinfection of wounds in animal hosts.[12] Although horses and foals are the traditional hosts, the infection has been documented in other animals, including sheep, cattle, cats, dogs, wild birds, and humans.[12]
After the first reported human case in 1967, only 12 more cases were reported for several decades. A significant rise in human R equi infections was noted, which correlates with the spread of HIV infections.[12] Presently, it is recognized as an opportunistic infection, posing a significant risk for individuals with compromised immune systems. Case reports documenting significant morbidity in patients with suppressed immune systems continue to emerge.[13]
The incidence and prevalence of R equi as a human pathogen are remarkably low. This can be attributed to its primary occurrence in immunocompromised individuals and the necessity for particular exposure to infect humans. Some studies suggest that R equi might be underreported as a cause of pneumonia because, in gram staining, it appears as a "diphtheroid."[5] Furthermore, the organism may be disregarded as a pathogen in culture, as it may appear as part of "normal respiratory flora."[5]
Pathophysiology
R equi is classified as Nocardioform actinomycetes within the Actinobacteria genus.[14] A rod-to-coccus growth cycle variation and the presence of cell wall mycolic acids characterize these bacteria.[14] They exhibit robust growth on nonselective media at 37 °C and form large, smooth mucoid colonies. Although the early colonies, which are less than 4 days old, lack pigmentation, the colonies gradually develop a distinctive salmon-pink color after 4 to 7 days of incubation. However, some may remain nonpigmented or display a slight yellow hue.[14]
The organism is gram-positive and, in liquid media, appears as long rods or short filaments, which may or may not exhibit branching.[14] When grown on Löwenstein–Jensen agar, it may exhibit acid-fast characteristics. R equi can be differentiated from corynebacteria by its non-carbohydrate fermenting nature. In addition, the bacteria are gelatinase-negative, catalase-positive, urease-positive, and oxidase-negative.[14]
R equi infects macrophages and appears to evade killing by interfering with phagosome-lysosome fusion.[14][15] The pathogenicity depends on the presence of Rhodococcus virulence-associated plasmid (vap) genes. Strains containing virulence-associated protein A (VapA) are highly virulent, whereas those containing virulence-associated protein B (VapB) are considered to have intermediate virulence. Strains lacking either of these plasmids are considered avirulent. However, a recent case series of R equi infections in patients with HIV reported that in more than 50% of the cases, virulence plasmids were not detected.[16] Recent studies have identified a plasmid encoding virulence-associated protein N (VapN) in R equi isolates from humans.[17] The significance of this plasmid in the epidemiology and pathogenesis of R equi infections in humans remains to be elucidated.[17]
Typical findings include necrotizing granulomatous reactions with granular macrophages that stain positively with periodic acid–Schiff (PAS).[18] In addition, macrophages containing gram-positive coccobacillus bacteria may be observed.[18] This granulomatous reaction may lead to chronic cavitary pneumonia, which can resemble pulmonary tuberculosis.[4]
R equi infections can develop chronic granulomatous inflammation called malacoplakia, particularly in immunocompromised hosts. Malakoplakia is characterized by an accumulation of PAS-positive histiocytes containing lamellated iron and calcium inclusions. Although these nodules may raise concerns about neoplastic processes, histological evaluation reveals malacoplakia, and R equi can be identified through gene sequence analyses.[19] Although malakoplakia is highly suggestive of R equi infection, it is not pathognomonic.
History and Physical
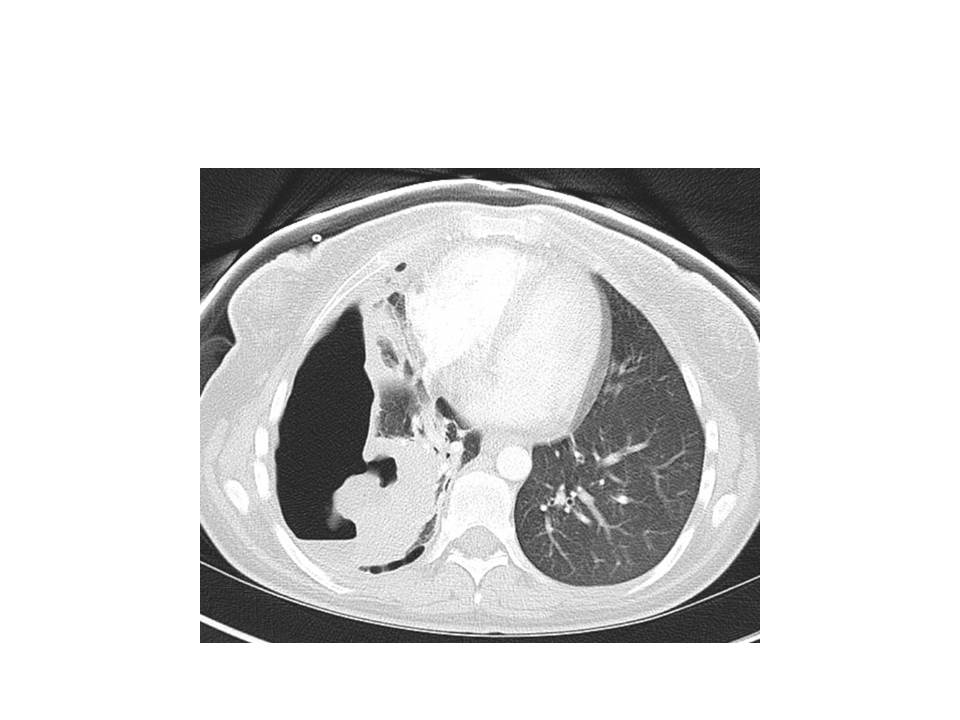
Pulmonary infections are the most common manifestation of R equi disease in humans. Data from a large cohort case series revealed that in individuals infected with this organism, pneumonia was identified in 76% of the patients, with the lung being the exclusive site of involvement in 82% of the cases.[20]
Typical symptoms of a subacute pulmonary infection typically manifest as the presenting symptoms, particularly in immunocompromised hosts, with fevers, cough, chest pain, fatigue, and weight loss. The presence of hemoptysis may vary. Cavitary lesions and pulmonary effusions are frequently observed. Pulmonary malakoplakia is highly suggestive of R equi infection, although not pathognomonic.[19] A case series involving immunocompromised patients reported pneumonia and bacteremia as the most common presentation.[21]
Localized infections in individuals without underlying pulmonary disease can lead to septic arthritis, cellulitis, and meningitis. Data regarding these manifestations are limited to case reports. The most common radiological findings include ill-defined pulmonary consolidation and irregular areas of cavitation, with a preference for the upper lobes.[5]
Evaluation
Although R equi can readily grow in microbiological cultures, it is crucial to maintain appropriate clinical suspicion and ensure effective coordination and communication among clinicians and laboratory personnel. This is essential due to the close resemblance of Rhodococcus to tuberculosis, Nocardia, and certain Corynebacteria, making it relatively easy for the laboratory to misdiagnose Rhodococcus as "normal respiratory flora" initially. Suitable culture materials include blood, sputum, pleural fluid, abscess aspirate, bronchoalveolar lavage fluid, peritoneal fluid, cerebrospinal fluid, wounds, lymph nodes, and tissue culture from any suspected affected organ.[5]
As most patients are immunosuppressed, the isolation of a gram-positive coccobacillus or acid-fast organisms from an immunocompromised patient with cavitary lung disease should raise suspicion for R equi infection.[5] Blood cultures should always be obtained, especially in immunocompromised individuals, as they are frequently positive.[21]
Treatment / Management
Without guidelines and comprehensive data, the clinical management of this infection relies on veterinary data, case reports, and in vitro studies. For most Rhodococcus infections, the following general treatment principles should be noted:
- Frequent use of multiple agents, especially in immunocompromised patients.
- If the pathogen is susceptible, beta-lactams (except carbapenems) should be avoided due to the risk of resistance developing during therapy.
- Prolonged therapy is often necessary.
- Improvement of host immunity is an integral part of a successful treatment outcome.
Antibiotics that have shown efficacy against this organism include extended-spectrum macrolides, rifampin, fluoroquinolones, aminoglycosides, glycopeptides (such as vancomycin), linezolid, and imipenem.[22] Almost all isolates of R equi are susceptible to vancomycin.[5] In addition, more than 90% of the isolates are susceptible to macrolides, carbapenems, ciprofloxacin, and rifampin.[5] However, most isolates generally resist trimethoprim-sulfamethoxazole, ampicillin-sulbactam, and clindamycin.[5]
A single agent, such as extended-spectrum macrolide or fluoroquinolone, may be sufficient for immunocompetent hosts. In contrast, using 2 or more agents for immunocompromised hosts is preferable, with at least 1 demonstrating good macrophage penetration. Antibiotic options include vancomycin, linezolid, carbapenems, fluoroquinolones, aminoglycosides, macrolides, and rifampin. When the central nervous system (CNS) is involved, selecting multiple agents with good CNS penetration is favored.[23][24][25][26]
The typical treatment for immunocompromised hosts with a severe infection involves intravenous therapy for 2 to 3 weeks, followed by oral therapy for a minimum of 2 to 6 months.[5] Definitive treatment should be followed by suppressive therapy in immunocompromised hosts, which generally includes rifampin with either a macrolide, quinolone, or doxycycline.[5]
Differential Diagnosis
Differential diagnoses of cavitary lung lesions include tuberculosis, granulomatosis with polyangiitis (GPA, formerly Wegener Granulomatosis), squamous cell carcinoma of the lung, Mycobacterium avium complex (MAC), nocardiosis, histoplasmosis, blastomyces infection, coccidioides infection, paracoccidioides, and aspergillus infection.[27]
Prognosis
The immunological status of the host is the primary determinant of treatment success and prognosis.[5] In immunocompromised hosts with underlying HIV infection, the absence of antiretroviral therapy is associated with a higher mortality rate from this infection. Patients with untreated HIV have a relative risk of death from this infection, 53 times greater than those who received appropriate antiretroviral therapy.[5] In contrast, immunocompetent hosts typically exhibit an excellent prognosis, even with a short course of antibiotic therapy.[5]
Complications
Data regarding complications of this infection are limited to case reports and case series, with the majority dating back several decades. The most prevalent complications include bacteremia and subsequent sepsis.[5] Disseminated R equi infection has been reported to cause CNS disease with brain abscesses, mediastinal and intra-abdominal lymphadenopathy, osteomyelitis, and spondylodiscitis.[28]
Deterrence and Patient Education
In individuals with HIV, the most effective approach to primary prevention is the early initiation of antiretroviral therapy to restore immunological function.[29] Suppressive therapy is recommended for patients with immune dysfunction for secondary prevention, as described above.
Pearls and Other Issues
Although rare, infections caused by R equi are recognized as an important cause of cavitary lung infections, especially in patients with HIV and other immunocompromising disorders.
Proven links with horses are not always evident, and some reports and case series have not identified any such associations. In these situations, other animals (non-horses) could have been implicated, and the infection becomes unmasked with the suppression of cellular immunity in the host.
Relapse of Rhodococcus infection, even after a complete course of therapy, has been reported and may occur during suppressive therapy. Relapse may occur at the same site as the initial infection or at a distant site. Linezolid has been successfully used to treat relapse.
In summary, R equi is a rare cause of subacute, necrotizing infection, mostly cavitary pneumonia and lung abscess, especially in the immunocompromised host. A prompt diagnosis and directed therapy, often involving 2 or more agents, are usually necessary for these patients to achieve a favorable outcome.
Enhancing Healthcare Team Outcomes
Although Rhodococcus infections are rare, they pose a significant risk of morbidity and mortality if the diagnosis is overlooked. Moreover, routine testing may not always identify the causative organism in respiratory fluid. Clinicians must maintain a high degree of suspicion for this disease, especially in immunocompromised individuals presenting with cavitary pulmonary lesions. In addition, they should promptly alert laboratory personnel to ensure adequate testing is conducted. Once the infection is confirmed, adequate therapy with prolonged duration is indicated in most individuals.
A clinical pharmacist plays a crucial role in ensuring patients complete oral therapy and minimizing potential drug-drug interactions. Simultaneously, a clinical nurse is essential for educating patients on medication compliance and limiting exposure to potentially infected materials or environments. A well-coordinated healthcare team, including clinicians, nurses, pharmacists, and laboratory personnel, is crucial for early diagnosis, effective treatment, and improved clinical outcomes.