Continuing Education Activity
Glioma is the most common form of central nervous system (CNS) neoplasm that originates from glial cells. In the United States, there are six cases of gliomas diagnosed per 100,000 people every year. Gliomas are very diffusely infiltrative tumors that affect the surrounding brain tissue. Glioblastoma is the most malignant type while pilocytic astrocytomas are the least malignant brain tumors. This activity reviews the pathophysiology of gliomas and highlights the role of the interprofessional team in its management.
Objectives:
- Describe the different types of gliomas.
- Review the pathophysiology of gliomas.
- Summarize the treatment of gliomas.
- Explain modalities to improve care coordination among interprofessional team members in order to improve outcomes for patients affected by gliomas.
Introduction
Glioma is the most common form of central nervous system (CNS) neoplasm that originates from glial cells. In the United States, there are six cases of gliomas diagnosed per 100,000 people every year. Gliomas are very diffusely infiltrative tumors that affect the surrounding brain tissue. Glioblastoma is the most malignant type while pilocytic astrocytomas are the least malignant brain tumors.
In the past, these diffuse gliomas were classified into different subtypes and grades based on histopathologies such as a diffuse astrocytoma, oligodendrogliomas, or mixed gliomas/oligoastrocytomas. Recently, gliomas were classified based on molecular and genetic markers.[1] These advances have more specific prognostic and therapeutic benefits for patients with gliomas. In addition to molecular and genetic markers, gliomas are classified in grade I to IV based on the degree of proliferation indicated by the mitotic index and the presence or absence of necrosis.
Etiology
There are three common types of gliomas, which are classified based on the phenotypic cell characteristics: astrocytomas, ependymomas, and oligodendrogliomas. These cell gliomas are further classified to low grade, atypical, and high-grade tumors based on cell morphology, mitotic activities, and molecular marker. The World Health Organization (WHO) grading system utilizes molecular markers that have shown to have significant prognostic and therapeutic implications.[2]
- Astrocytomas: Originated from astrocytes and can be encapsulated, preserving clear borders between normal and tumor cells, or infiltrative, indicating advanced grade. Low grades are common in children while high grades are common in young adults and older patients.
- Oligodendrogliomas: Originated from oligodendrocyte cells. These are less infiltrating than astrocytomas and are common in adults.
- Ependymomas: Originated from ependymal cells which are found lining the ventricular cavities and the central canal of the spinal cord. These are common in the pediatric patient population.
Epidemiology
There is an estimation of 80,000 newly diagnosed cases of primary brain tumor each year in the United States. Around one-fourth of which (i.e. 20,000) are gliomas. The total number of glioblastomas diagnosed each year is around 12,000 cases (approximately 15% of total newly diagnosed brain tumors).
Pathophysiology
Headaches are the most common initial presenting symptom of patients with glioma. The pathophysiology of headaches is theorized to be the result of tumor growth that places a mass effect on surrounding tissue. The mass effect, in turn, leads to pressure in the microvasculature and leads to edema. Depending on the location of the tumor in the brain, the mass effect leads to signs of a brain tumor. For example, frontal lobe tumors can present with behavioral changes while dominant temporal lobe tumors can present with receptive speech problems. Other symptoms related to mass effects include nausea, vomiting, and change in vision, Seizures are the second most common symptom of presentation. The pathophysiology of seizures is attributed to tumor irritation to the cerebral cortex that leads to focal or generalized seizures. Other presenting symptoms of gliomas are tingling sensations, weakness, difficulty ambulation, and in rare cases, patients can present in a comatose state due to hemorrhage within the tumor which leads to an acute herniation syndrome.
Toxicokinetics
There are some epidemiological studies suggesting that ionizing radiation and some radiofrequency waves can increase the chance of redeveloping high-grade gliomas. However, these studies lack specificity to gliomas. However, advances in the molecular biology and genetics of gliomas have revealed that low-grade gliomas transform into high-grade gliomas by altering the genetic makeup of low-grade gliomas. Therefore, one can hypothesize that environmental and treatment-related toxicokinetics can play a role in the transformation of low-grade gliomas.
History and Physical
The most common presentations in brain gliomas are headaches, nausea, vomiting, seizures, and in more advanced cases weakness or altered mental status. The neurological examination of these patients can be normal or present different degrees of focal weakness, sensory deficits, or in a severe situation altered mental status due to an acute mass effect resulted from the tumor swelling.
Evaluation
The following imaging diagnostics are appropriate in the setting of history and physical examination suggestive of brain tumors:
- Computed tomogram (CT) head: A good study should be done to evaluate acute intracranial findings such as hemorrhage, edema, and asymmetry in the brain tissue.
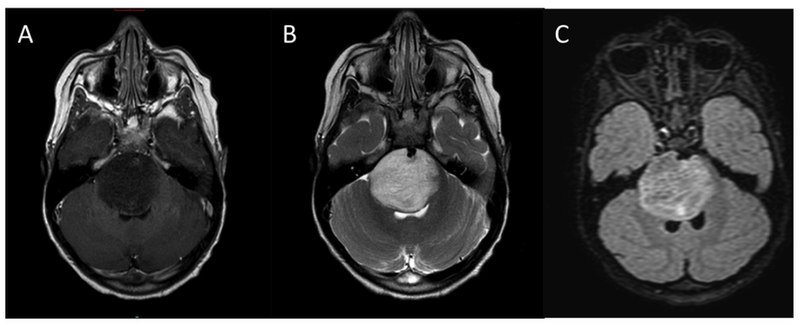
- Magnetic resonance imaging (MRI) Brain: This is a sensitive study to determine the characteristics of brain tumors. Low-grade tumors usually do not enhance while high-grade tumors have a different degree of enhancement. In addition, MRI is a great study to evaluate the degree of cerebral edema. An MRI study of the brain will facilitate the treatment plan and response to surgical, radiation, chemotherapy.
- Chest X-Ray: Patients newly diagnosed with a brain tumor need an appropriate metastatic workup based on the patient's history and physical examination. For example, patients with an extensive history of tobacco use might require a chest x-ray. If there is any finding of a mass on the chest x-ray, it needs to be investigated using a CT scan of the body or positron emission tomography (PET) scan to identify the potential primary source for metastatic disease.
Treatment / Management
The WHO classification of gliomas is used to guide glioma treatment. As indicated in the classification, most patients require surgical intervention via gross total resection or biopsy.[3]
2016 WHO Grades of Gliomas
1. Diffuse astrocytic and oligodendroglial tumors[4][5][4]
- Diffuse astrocytoma, IDH-mutant (Grade II)
- Anaplastic astrocytoma, IDH- mutant (Grade III)
- Glioblastoma, IDH wild-type (Grade IV)
- Glioblastoma, IDH wild-type (Grade IV)
- Oligodendroglioma, IDH-mutant, and 1p/19q-co-deleted (Grade II)
- Anaplastic Oligodendroglioma, IDH-mutant, and 1p/19q-co-deleted (Grade II)
2. Other astrocytic tumors
- Pilocytic astrocytoma (Grade I)
- Subependymal giant cell astrocytoma (Grade I)
- Pleomorphic xanthoastrocytoma (Grade II)
- Anaplastic pleomorphic xanthoastrocytoma (III)
3. Ependymal tumors[6]
- Subependymoma (Grade I)
- Myxopapillary ependymoma (Grade I)
- Ependymoma (Grade II)
- Ependymoma, RELA fusion-positive ( Grade II or III)
- Anaplastic ependymoma (Grade III)
4. Other gliomas[7][8][9][8]
- Angiocentric glioma (Grade I)
- Chordoid glioma of the third ventricle (Grade II)
Treatment of Gliomas
1. Surgery:
- Grade I: These gliomas are surgically curable.
- Grade II: A safe gross total resection and radiographic follow-up are acceptable current practices.
- Grade III: A safe gross total resection, concomitant chemoradiation, and radiographic follow-up for recurrence are an acceptable treatment.
- Grade IV (glioblastoma): A safe gross total resection, concomitant chemoradiation, and radiographic follow-up for recurrence are an acceptable treatment.
2. Chemoradiation: Currently, Stupp protocol is a standard of care for Grade III-IV gliomas. The protocol consists of radiotherapy and concomitant chemoradiation using a total of 60 Gray to 2 Gray per daily fraction over 6 weeks and temozolomide.
3. Treatments for Recurrence: Options for recurrent gliomas include re-operation with Gliadel wafers and targeted therapy such as angiogenesis inhibitors or immunotherapy. The effectiveness of all these adjuvants therapies is in development.
4. Others Treatments: High-grade glioma patients are prone to seizures, malignant edema, and complication related immobility. Therefore, these patients need antiepileptic medications, deep venous thrombosis (DVT) prophylaxis, and steroids before, during, and after the course of treatments to avoid cerebral edema.
Differential Diagnosis
The differential diagnoses include:
- Abscess
- Demyelination
- Gliosis
- Infarct
- Metastasis
Prognosis
The prognosis of gliomas depends on several factors including:
- Age of the patient
- Comorbidities
- Grade and location of the tumor
- Presence of hydrocephalus
- Response to adjuvant therapy
- The extent of surgical resection
Complications
- Increased intracranial pressure
- Seizures
- Hydrocephalus
- Brain herniation
- Hemorrhage into the tumor
- Local spread
- Spinal metastases
- Death
Deterrence and Patient Education
The patients should be educated on the importance of regular follow-up visits, having medications on time, and regarding driving restrictions.
Pearls and Other Issues
Gliomas in general, specifically glioblastomas, are very difficult to treat. Despite advances in understanding the molecular biology and genetics of gliomas, no significant impact has been made toward preventing the lethality of high-grade gliomas. Therefore, there is a continuing need for clinical and basic science investigations to advance the care of this lethal disease.
Enhancing Healthcare Team Outcomes
Patients with gliomas require an interprofessional treatment approach. Neurosurgeons, neuro-oncologists, radiation oncologists, and other health professionals will play to enhance patient care and optimum outcome.[10]