Continuing Education Activity
Spinal dysraphism is a congenital abnormality that results in an abnormal structure in the spine, including the bony structure, the spinal cord, and the nerve roots. Myelomeningocele is a spinal dysraphism in which the spinal cord and its contents herniate through a congenital bony defect in the posterior elements. This activity reviews the evaluation and management of spinal dysraphism and myelomeningocele and highlights the role of the interprofessional team in evaluating and treating patients with this condition.
Objectives:
- Identify the etiology of spinal dysraphism medical conditions and emergencies.
- Review the appropriate evaluation of spinal dysraphism and myelomeningocele.
- Outline the management options available for spinal dysraphism and myelomeningocele.
- Describe interprofessional team strategies for improving care coordination and communication in patients with spinal dysraphism and myelomeningocele to improve outcomes.
Introduction
Spinal dysraphism encompasses congenital problems that result in an abnormal bony formation of the spine and/or the spinal cord. It is caused by the maldevelopment of the ectodermal, mesodermal, and neuroectodermal tissues. The two major types of spinal dysraphism are based on the appearance, i.e., aperta (open) if the lesion is visible and occulta (closed) if the lesion is not visible on the surface.[1] Common manifestations are meningocele, myelomeningocele, lipomeningocele, lipomyelomenigocele, myeloschisis, and rachischisis.[1]
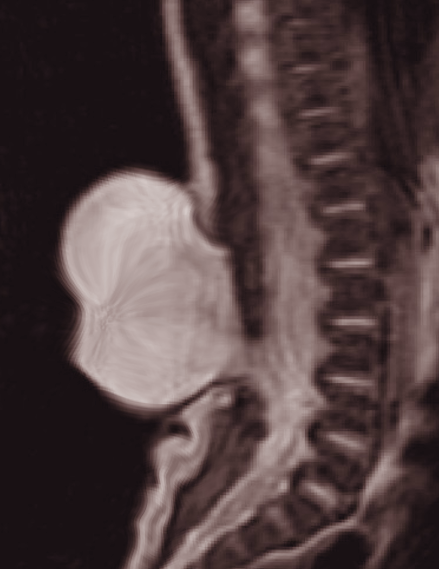
This condition also correlates with cutaneous conditions including port-wine stain, hemangioma, hypertrichosis, fibroma pendulum, pigmentary nevus, lipoma, dermal sinus, and deviation of the gluteal furrow. Myelomeningocele is a spinal dysraphism in which the spinal cord and its contents herniate through a congenital bony defect at the posterior elements of the spine, in most cases, the spinous process.[2] It falls under the aperta category and is also known as an open neural tube defect. This is a serious malformation and is associated with a high mortality rate. Myelomeningocele is the most common presentation of spinal dysraphism and constitutes about 80% of the cases.[3][4]
Etiology
The etiology of spinal dysraphism is multifactorial.[5] In cases of myelomeningocele, perinatal folic acid intake has shown to reduce the incidence of myelomeningocele significantly. Other potential etiologies include genetic inheritance and racial backgrounds. Usually, infants who are born with spinal dysraphism are born to mothers who have given birth to children with no history of spinal dysraphism. However, after one child with spinal dysraphism is born, the risk will increase to 1 in 20 for subsequent childbirth. Some infants born with spinal dysraphism can have chromosomal anomalies as trisomies, duplications, deletions, and some single-gene mutations.[6] Some reports show that pregestational diabetes increases the risk of central nervous system malformations, including spinal dysraphism.[4]
No specific individual factor has been identified to be related to aperta and occulta type.[4] Nutritional deficiency is the most common risk factor reported for any kind of spinal dysraphism, causing up to 50% of all cases. In addition to folic acid deficiency, zinc deficiency is also found to be associated with this condition. Excessive intake of some elements can also lead to spinal dysraphism. Vitamin A deficiency or excess can be associated with the formation of spinal dysraphism. Moreover, nitrates are present in canned meat and groundwater and are known to cause this condition. Cytochalasin is a fungal metabolite, and its intake can also cause spinal dysraphism in children.[4] Some medications are also found to be associated with spinal dysraphism. For example, sodium valproate, a well-known antiepileptic drug, is related to 1% to 2% chances of myelomeningocele formation when used in pregnancy.[7][8]
Epidemiology
The incidence of all forms of spinal dysraphism (open and closed) is 0.5 to 8 cases per 1,000 live births.[9] There are significant geographic variations. As expected, developing countries have a higher incidence. However, the incidence has decreased over time due to improved nutrition and awareness about folic acid intake before and during pregnancy.[1] In the United States, a lower incidence is found with 0.3 to 0.4 cases per 1,000 live births due to the mandatory folic acid fortification of cereals and improved prenatal education.[4][10] Myelomeningocele occurs in approximately 1 in 1200 to 1400 births.[4] The incidence is not higher in any specific ethnic group; however, females have a slightly higher incidence as compared to males.[4]
This condition most commonly affects the lumbar and sacral region of the spine and, least commonly, the cervical spine. About 0 to 5% affects the cervical spine, 5% to 10% the thoracic spine, 20% to 30% the thoracolumbar spine, 20 to 30% the lumbar spine, 30 to 50% the lumbosacral spine and 5 to 15% the sacral spine.[11] Cervicothoracic spinal dysraphism is relatively rare, with an incidence of 1% to 6.5%.[12]
Pathophysiology
Spinal dysraphism results from abnormal cell migration and differentiation of the neural tube during the first trimester of pregnancy. The formation of the neural tube involves primary neurulation and a secondary neurulation process. During primary neurulation, the neuroectoderm folds with the last caudal portion closing approximately on day 27. At the same time, the mesoderm migrates and forms the posterior vertebral elements, and the ectoderm migrates, forming the skin.
The pathophysiology of myelomeningocele is a failed closure of neural tube during primary neurulation producing a cystic mass of neuronal elements including dural, arachnoid, spinal cord, nerve roots, cerebral spinal fluid through the bone and skin defect.[13] Secondary neurulation, which starts around day 28, promotes further neural development forming the caudal spine and filum terminale.[13] A defect at this stage produces closed neural tube defects. Problems with premature disjunction where the neural tube prematurely separates from the overlying ectoderm prior to neural tube closure may form spinal dysraphism associated with lipomas like lipomeningocele, lipomyelomenigocele or spinal cord lipomas.[13] Failures of primary disjunction permit connections between the ectoderm and neuroectoderm and usually manifested as dermal sinus.[13]
Several types of spinal dysraphisms are found. Spina bifida cystica is a type of spinal dysraphism in which a cyst protrudes through a bone defect of the posterior elements of the spine. The content of the cyst may include dura, arachnoid, spinal cord, and/or spinal nerves and associated spinal fluid. It occurs most commonly in the lumbar or lumbosacral spine. Myelomeningocele, meningocele, myeloschisis are the different types of spina bifida cystica, the former being the most common. Spina bifida occulta is a type of spinal dysraphism in which there is a bony defect in the spine, commonly in the lumbar spine, but usually, there is no visible skin anomaly. It is found in a significant number of healthy adults and is usually identified in imaging studies. Syringomeningocele is a type of spinal dysraphism in which there is a spinal fluid-filled central canal of the spinal cord surrounded by a thin membrane. Syringomyelocele is a spinal dysraphism in which the spinal cord tissue expands with a thin-walled central canal.
Isolated vertebral defects are the most common and least severe form of occult spinal dysraphism. Other examples include neurenteric cysts, split notochord syndrome, split spinal cord malformation, sacral meningeal cysts, spinal lipomas, caudal regression syndrome, dorsal dermal sinus tracts and cysts, and tethered cord syndrome.[9] The term tethered cord syndrome was coined in 1976 for a condition previously called filum terminale syndrome.[14]
History and Physical
In most cases, open spinal dysraphism or myelomeningocele is diagnosed in utero or at birth. The symptoms differ for open and occult spinal dysraphism. If an open spinal dysraphism was not diagnosed in utero, during delivery, a midline cystic mass in the back is identified with a variable degree of neuronal element protruding through the bone and skin defect. The skin over the lesion is usually not well-developed and can get scraped off during delivery. This leads to cerebrospinal fluid leak and possible exposure of the spinal cord contents. Due to this exposure, the patient can develop infections like meningitis.[15]
Infants with spina bifida cystica present with lethargy, poor feeding, irritability, stridor, ocular motor incoordination, and/or development delay. Older children may show cognitive or behavioral changes, decreased strength, increased spasticity, changes in bowel or bladder function, lower cranial nerve dysfunction, back pain, and/or worsening of spinal or lower extremity deformities.[16] Occult spinal dysraphism might not be detected early in life. Many patients only have cutaneous lesions like a tuft of hair, port-wine stain, or sinus that leads to further investigations and diagnosis. Dermal sinus and diastematomyelia are common lesions that can be seen in occult spinal dysraphism. Patients with dermal sinus can develop meningitis that prompts further investigations. Another important manifestation in occult cases is tethered cord syndrome.[17][16] Symptoms of tethered cord syndrome include back pain, shooting leg pain, urinary dysfunction, and fecal incontinence. These symptoms can manifest in childhood or adulthood.[18]
On physical examination, motor and sensory dysfunction should be assessed, focusing on neuro-segmental levels based on muscle strength of specific nerve roots.[19] Motor weakness can be asymmetric and might not correspond to the sensory level. Patients with myelomeningocele are categorized based on the spinal segment affected. In tethered cord syndrome, different cutaneous findings can be seen on the physical examination. There is usually a midline cutaneous lesion in the lumbosacral region. Sometimes, there is only a cutaneous dimple in the midline above the gluteal cleft. Neurological examination may show motor weakness, a sensory deficit in the lower body, or decreased anal tone.[20]
Evaluation
Some several diagnostic tests and investigations can be performed to diagnose spinal dysraphism. They are particularly important in diagnosing the occult type because it mostly presents with a skin lesion, and early diagnosis is important to limit permanent disabilities.
Amniocentesis is a prenatal study in which the amniotic fluid is analyzed. An elevated alpha-fetoprotein (AFP) in the amniotic fluid may suggest a neural-tube defect. This is usually performed at 16 to 18 weeks of gestation and is a screening test that is routinely performed in many countries. An elevated AFP level may prompt further investigations.[21] Fetal ultrasonography is another screening test. It the AFP is elevated, the diagnosis can be further confirmed with fetal ultrasonography. Lemon shaped head, banana cerebellum, and ventriculomegaly can be observed on ultrasonography in those cases with spina bifida. These tests allow caregivers to access appropriate services and enhance patient care. These advanced imagining modalities can help assess if the involvement is compatible with life or not and can help consider pregnancy termination.[22] Ultrasonography can be employed to evaluate soft tissue masses as it has good sensitivity in the diagnosis of spinal cord abnormalities in children with a neurogenic bladder.[23] Lumbar X-ray is a good first screening tool to visualize the bony structure of the spine. It shows the level of lesion and bone splitting.[24]
Computed tomography scan of the brain and spine is used to evaluate for associated hydrocephalus that can be present in some patients of spinal dysraphism.[25] Magnetic resonance imaging is used for assessment of the spine, spinal cord, associated anomalies, and the brain. It is the most trusted tool in diagnosing spinal dysraphism due to its superior soft-tissue imaging. It is the best study to show spinal cord malformations, including Chiari II and tethered cord.[26]
Children with isolated coccygeal or intergluteal dimples or pits do not require imaging because the associated risk of having occult spinal dysraphism is 0.34%, whereas those with lumbosacral dimples or pits have a 3.8% risk. Children with lumbosacral dimples located at a distance greater than 25 mm from the anus or with lesions that are greater than 5 mm in size should undergo imaging due to their higher probability of occult spinal dysraphism than children with isolated coccygeal dimples.[9]
Psychometric tests are used for the assessments of intelligence and cognitive function. They are indicated for patients with hydrocephalus and for patients who display deficits in speech and language functions and/or cognitive or academic skills.[27]
Treatment / Management
The initial intervention for a patient with myelomeningocele includes intravenous antibiotics, neurosurgical closure of the defect within 24 to 48 hours of birth to prevent infections and later complications, and ventriculoperitoneal shunt placement if the patient develops hydrocephalus. If the infant is in a critical status secondary to respiratory failure, sepsis, or other gross congenital organ anomalies causing life-threatening problems, initial supportive care is recommended.[28] The surgical procedure involves reversing the failed steps of normal spinal cord closure employing extensive knowledge of normal anatomy and embryology.[16] It is carried out under general anesthesia with an endotracheal tube. In the first step, the neural placode is separated, tubularized, and then stitched. After that, the dura is separated from the underlying tissues and closed. Then the skin is approximated and sutured.[1] Postoperative care has a vital role in the success of the surgery. The patient should be given prophylactic antibiotics, painkillers, and good nutrition. It is important to ensure that patient is nursed in a prone position to avoid aspiration and wound dehiscence. Wound care is essential to avoid wound infection. Dressing change and avoiding urinary and fecal contamination of the wound is necessary. The patient should also be monitored and treated for urinary problems. [8] Recently, specialized centers are performing fetal myelomeningocele closure. This reduces the development of hydrocephalus, but at the risk of an increase in prenatal complications and preterm birth.[16] Meningocele repair should be performed in an elective setting as the skin is usually intact. Surgical repair is followed by a lifetime follow-up by a multidisciplinary team.[29]
Occult spina bifida usually does not require surgery. Closed neural tube defects may require surgery if the patient presents symptoms. Untethering surgery is performed if the patient develops new or progressive symptoms. Some surgeons recommend doing prophylactic surgery for the tethered cord to prevent irreversible urinary problems when they occur.[4] In those cases of tethered cord, surgery involves removing the structure anchoring the cord, or cutting the film terminale so the cord can ascend and release the tension on the neural elements. The majority of patients with myelomeningocele have radiological evidence of tethered cord but do not need surgery.[4] Those with new symptoms or progressive symptoms will require surgery.
Many children with hydrocephalus will require shunt revisions throughout their lifetime. Patients with symptomatic Chiari II malformations will require suboccipital decompression. If they have a ventricular shunt, it must be checked to assure it is working adequately before doing a suboccipital decompression.[30] The development of a spinal cord syrinx may point to tethering of the cord, and appropriate treatment should be performed.
Differential Diagnosis
- Sacrococcygeal teratomas
- Benign teratomas
- Subcutaneous lipomas
- Lymphangiomas
- Cystic teratomas
- Spinal epidural abscess
- Spinal cord masses
- Pilonidal cyst
- Inclusion dermoid
- Caudal regression syndrome
Prognosis
The prognosis of spinal dysraphism varies from case to case. It depends on many factors, such as the extent of neurological defect, presence of congenital malformations, time to treatment, and level of care. Usually, lower and less severe lesions have a better outcome as compared to higher lesions with hydrocephalus. Patients with lower and smaller lesions can be ambulatory. The majority of patients with myelomeningocele have normal intelligence, although 60% have some learning disabilities.[4] Those with higher lesions tend to develop significant hydrocephalus and do not perform well academically. Most children with myelomeningocele require lifelong treatment focused on the damaged spinal cord and nerves. Children are usually followed closely with biannual clinic visits during childhood and annually during adulthood.[4]
The life expectancy of patients suffering from spinal dysraphism depends on the size of the lesion. Patients with large complex defects have a shorter life expectancy. According to statistics, 40% to 50% of children with severe defects die as infants. Patients with higher and smaller lesions and no hydrocephalus have a longer life expectancy. Renal failure is the most common cause of death among these patients.[4] The life expectancy of these patients has improved greatly with time owing to better healthcare services. However, most of these patients remain dependent on their parents and caretakers even in adulthood.[4] Nowadays, the majority of patients with myelomeningocele have a near-normal life expectancy if they do not develop systemic complications.
Complications
Bladder dysfunction: Most patients with myelomeningocele have some degree of bladder incontinence. Preventive goals are directed toward preventing infection with the implementation of bladder drainage utilizing intermittent catheterization or indwelling catheters. Bladder stimulation has shown to improve bladder emptying and reduce infection.
Bowel dysfunction: Myelomeningocele is associated with anal sphincter dysfunction that results in bowel incontinence. Assisted bowel emptying reduces barriers associated with social activities, including attending school and personal relationships.
Immobility: Most myelomeningocele patients have significant weakness, which results in severe ambulation deficits or paraplegia. Bracing using external orthosis can help to maximize their mobility and ensure a near-normal developmental progression. In children over 1-year-old, utilizing a standing frame can reduce the risk of osteoporosis and the formation of contractures in lower extremities. A wheelchair can provide mobility for older children and adults.
Infections: Due to a neurogenic bladder, many have urine colonization and infections. Shunts are also prone to infections. When shunts are placed, infections can occur superficially at the skin or intraabdominal, as many of these patients have multiple abdominal procedures.
Postoperative and Rehabilitation Care
Rehabilitation for patients with myelomeningocele includes physical, occupational, recreational, and speech therapies. Patients with brainstem dysfunction have swallowing difficulties and may need swallowing evaluation with potential feeding-tube placement to prevent the risk of aspiration.[31]
Consultations
The interdisciplinary evaluation includes pediatric specialists in rehabilitation, neurosurgery, urology, plastic surgery, and orthopedic surgery. The disease is associated with local and systemic manifestations that require the involvement of more than one specialty. The collaborative efforts of specialists from different fields can improve the quality of life and prognosis of children with spinal dysraphism. Rehabilitation programs also affect the psychosocial aspect of this disease and make the social integration of these children easier. Genetic counseling is also recommended.
Deterrence and Patient Education
Because of the worldwide educational effort of the importance of folic acid intake before and during pregnancy, the incidence of myelomeningocele and neural tube defects has undergone a significant reduction. There is a need to continue educating patients during childbearing age to take perinatal vitamins that include folic acid.[32] Advocate for more comprehensive folate fortification in those countries without written standards, and thereby prevent the most common and severe birth defect from impacting the human brain and spinal cord.[33] Patients with spinal dysraphism should be encouraged to be very active at rehabilitation programs.
Pearls and Other Issues
Myelomeningocele can be associated with Chiari type II malformation, hydrocephalus, and tethered spinal cord.
Chiari type II malformation can present with lower brainstem and upper cervical spine compression symptoms, including pharyngeal paralysis, apnea, swallowing difficulty, respiratory stridor, nystagmus, upper extremity weakness.
Hydrocephalus can present with coordination dysfunction, headaches, lethargy, refractory seizures, and cognitive dysfunction.
Tethered spinal cord during childhood growth can present with worsening of a spinal deformity, new onset of hip dislocation, bladder dysfunction, bowel dysfunction, and new sensorimotor deficit.
The recommended intake of folic acid is 0.4 mg (400 mcg) per day for all women anticipating pregnancy. This should continue during pregnancy, at least during the first trimester.
Enhancing Healthcare Team Outcomes
Patients with spinal dysraphism, such as myelomeningocele, require interdisciplinary evaluation and treatments to provide patient-centered care and prevent complications related to the disease process. Interdisciplinary evaluation, including pediatric specialists in rehabilitation, neurosurgery, urology, and orthopedic surgery, is indicated. Interprofessional approaches have shown to have a significant impact on the outcome of these patients. Prevention is the most important and key element; therefore, state and country health departments, obstetricians, social workers, and prenatal nurses play an enormous role in the management of these conditions.