Continuing Education Activity
Peripheral intravenous (IV) lines, catheters or cannulas are indwelling single-lumen plastic conduits that allow fluids, medications and other therapies such as blood products to be introduced directly into a peripheral vein. Placement of peripheral lines is the most commonly performed invasive procedure in acute healthcare settings with more than 1 billion lines being used annually worldwide. This activity describes strategies for placement of peripheral IV lines by anatomical, landmark-based techniques, and highlights the role of the interprofessional team in placing and caring for IV lines while minimizing complications.
Objectives:
- Describe the indications for peripheral IV line placement.
- Outline the relative contraindications for peripheral line placement in certain clinical conditions.
- Summarize the venous anatomy of the arm for peripheral access.
- Explain the importance of a team approach to patients needing peripheral access and improving outcomes.
Introduction
Peripheral line placement, also referred to as peripheral intravenous (IV) cannulation, is the insertion of an indwelling single-lumen plastic conduit across the skin into a peripheral vein. Such devices may be referred to as peripheral IV (or venous) lines, cannulas, or catheters depending on the country.
They allow fluids, medications and other therapies such as blood products to be introduced directly into the cardiovascular system, bypassing other barriers to absorption and reaching most target organs very quickly. Once inserted, a well-functioning line can remain in use for several days if required, obviating the need for repeated needle insertion into the patient should ongoing treatment be needed. Placement of peripheral lines is the most commonly performed invasive procedure in acute healthcare settings with as many as 80% of hospital inpatients requiring intravenous access at some stage during their admission, and worldwide more than 1 billion lines are used annually.[1][2]
This article focuses on anatomical landmark-guided techniques for peripheral line placement. Lines may also be placed using real-time ultrasound guidance, which is particularly beneficial for those with suspected difficult access or multiple failed attempts at cannulation.[3]
Anatomy and Physiology
Various sites around the body can be successfully cannulated with a peripheral venous line. The non-dominant upper extremity is commonly chosen, because of comfort, reduced risk of dislodgement, and lower incidence of thrombosis or thrombophlebitis.[4]
- In the upper extremity, potential sites start distally with the metacarpal veins on the dorsum of the hand, which drain proximally through the dorsal venous arch, becoming the cephalic and basilic veins in the forearm. Near the antecubital fossa, these are connected by the median cubital and median antebrachial veins before continuing up the arm.
- In the lower extremity, lines may be placed starting with the dorsal venous plexus of the foot, which becomes the great and small saphenous veins in the leg.
- The scalp may be appropriate in neonates or infants, particularly where previous attempts at the limbs have failed or are likely to be unsuccessful. Frontal, occipital, superficial temporal or posterior auricular veins are an option.
Preferred veins are straight, distal and non-branched (venous valves are commonly near branching points). When using an access site on a limb, a tourniquet may be placed proximally to the site to engorge the vein, and it should feel spongy and non-pulsatile on palpation; veins that feel hard are more likely to be thrombosed, and pulsatile flow indicates an artery rather than a vein.
Identifying access sites may be more difficult in specific patient populations such as children, the obese, pregnant women, those with dark-toned skin, patients in shock, or those whose veins have been compromised by previous chemotherapy or by intravenous drug abuse.[5]
Indications
The most common indication is to allow the administration of IV medications and fluids. Lines are also commonly used for phlebotomy at the time of insertion (before administration of drugs or fluids which would dilute or contaminate the blood samples).
Contraindications
There are no absolute contraindications. Relative contraindications include coagulopathy; the presence of local infection, burns, or compromised skin at the intended site of insertion; and previous lymphatic nodal clearance, arteriovenous fistula formation, or deep venous thrombosis on the affected limb. In such cases, clinical judgment must be used to balance the benefits and risks of proceeding with line placement at that site.
Where an extended course of intravenous therapy will be required, another vascular access device such as a peripherally inserted central catheter may be more appropriate. These are more invasive and require specialist level expertise to place, but have lower failure rates in prolonged use. If using conventional peripheral lines in such settings, they may require frequent replacement.[6]
In time-critical cases with known difficult peripheral access or where multiple attempts at peripheral line placement have already failed, an ultrasound-guided technique may be necessary, or the clinician may consider using alternative routes of drug administration (such as oral, intramuscular, intraosseous, or central venous access).
Equipment
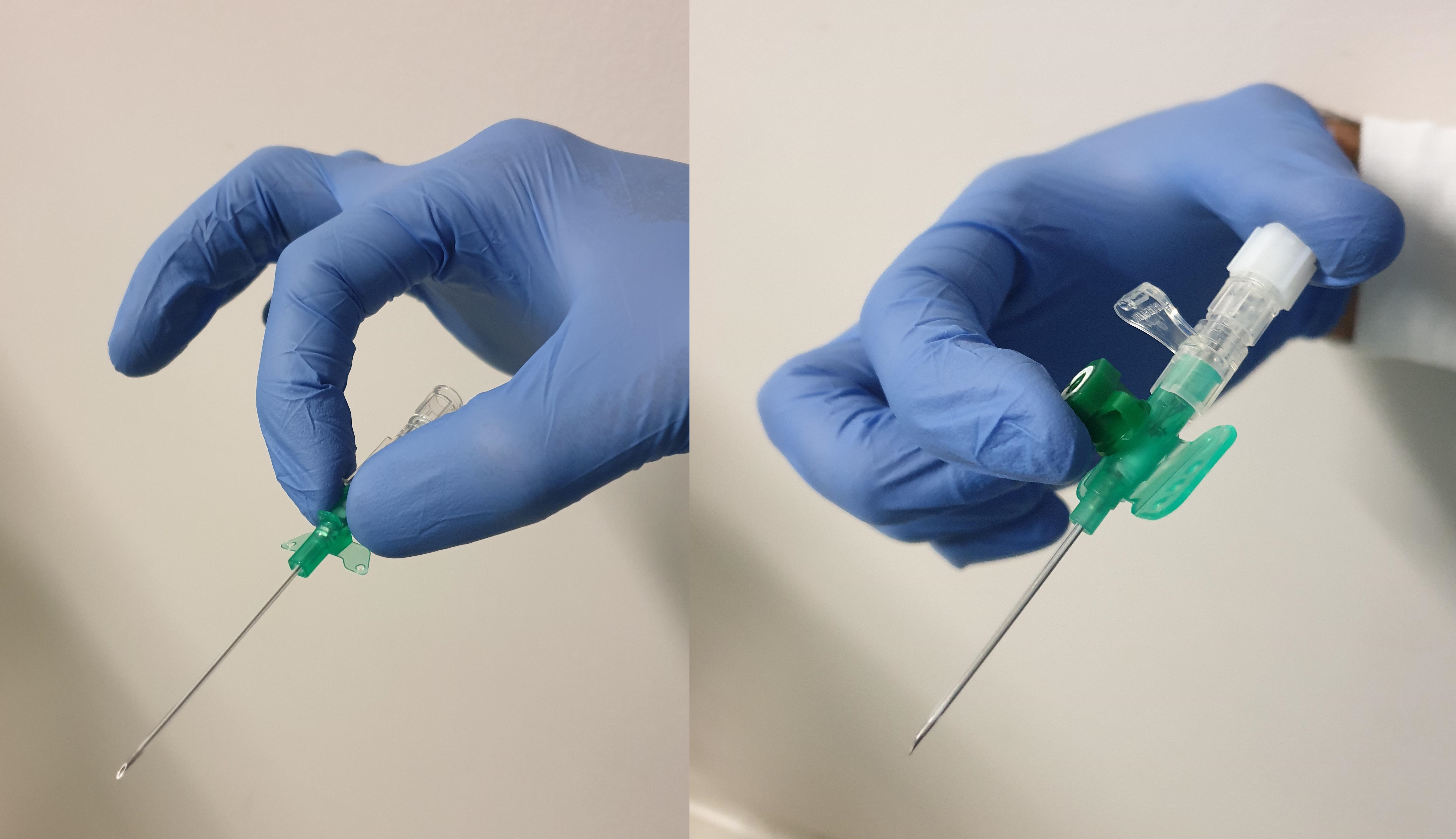
The IV line itself is a hollow, plastic, tube-shaped catheter that is attached to a larger hub which remains above the skin. Most modern lines are made of polyurethane, as this is thought to be less thrombogenic than older polyvinylchloride versions. The line is supplied pre-loaded over a hollow, laser-sharpened, beveled needle with a transparent "flashback" chamber at its opposite end. This allows the operator to identify when blood from the target vein starts to flow into the needle tip during insertion. The hub is color-coded according to the gauge of the needle, which reflects the internal diameter of the catheter and ranging in size from 14G to 24G depending on patient age and characteristics; the higher the gauge number, the narrower the catheter. The length of the catheter can vary between different manufacturers. Removing the needle from the external hub of the line reveals a standard Luer-taper connector to which a phlebotomy adapter, a needle-free injection bung, or an IV fluid administration set can be attached. The hub may also have a side port with a removable cap, allowing drug administration without disconnecting other ongoing IV fluids or medications.
So-called "safety lines" are a newer type of IV line with modifications intent on reducing accidental needlestick injury during line placement and have become more prevalent over the past decade.[7] They incorporate a mechanism (either active or passive) that covers the sharp end of the needle after withdrawing it from the hub of the cannula. Active safety lines require the user to press a button that pulls the needle into a plastic sheath, whereas passive safety lines automatically fold a small shield over the needle tip as soon as the needle is withdrawn from the hub.[8]
Aside from the cannula itself, other equipment required for peripheral vascular access includes antiseptic swabs or sponges, gauze, a needle-free bung, a prepared flush of sterile normal saline, and a sterile transparent moisture-permeable dressing. Local anesthetic agents may be of use with larger cannulas, or to minimize distress in selected patient cohorts such as young children. They can be infiltrated subcutaneously using a narrow-gauge needle shortly before the procedure (for example, 0.1 mL of 1% lidocaine). Alternatively, a bolus of topical ointment containing local anesthetic may be applied to the skin ahead of time and left in contact under a dressing.
Personnel
One operator is commonly sufficient for peripheral line placement, but the presence of an assistant may be beneficial for anxious or distressed patients, in children, or to help with optimal patient positioning in the case of challenging vascular access.
Preparation
When inserted for a specific procedure or treatment, the placement of peripheral lines should generally be as close to the procedure time as is possible to avoid the risk of the line becoming dislodged in the interim. The operator should perform hand hygiene and don single-use treatment gloves. If there has been an application of a topical local anesthetic ointment, this should be wiped off. A sharps disposal box should be close at hand.
A tourniquet is applied around the limb around 5 to 10 cm proximally to the site, tight enough to engorge the veins with blood but not so tight as to abolish arterial blood flow into the extremity.[4] This engorging will make it easier for the operator to locate veins and to successfully thread the catheter inside. The area is then inspected and palpated to identify a suitable vein before cleaning it with antiseptic. If infiltrative anesthesia will be in use, local anesthetic should be injected near the vein using a narrow needle to raise a small weal in the subcutaneous tissue at the site where the operator intends to pierce the skin with the line.; this will generally be slightly distal to where the operator intends to pierce the vein itself.
Technique or Treatment
The cannula should be gripped firmly in one hand with the forefinger and thumb on each side of the hub. Alternatively, if there is a side port, the tip of the forefinger may be curled around the cap of the side port with the thumb pressed over the end of the "flashback" chamber. The operator should use their other hand to apply distal traction, stabilizing the vein and insertion site with the surrounding skin stretched tautly. Needle insertion follows at a shallow angle of fewer than 45 degrees through the skin, aiming towards the vein and is advanced slowly until a "flash" of blood appears in the chamber. If only a drop of blood is visualized, part of the needle bevel may still be outside the vein, and the line should be flattened slightly and inserted incrementally further by 1 to 2 mm until good flow is observed. The cannula is advanced over the needle until the hub sits at the skin; a second "flash" of blood should appear within the catheter while it enters the vein.
The tourniquet is then loosened or removed. Using the other hand, the operator should apply pressure proximally over the vein and catheter; this prevents blood from running while the needle is withdrawn and discarded safely into a sharps disposal box. Any desired blood samples should be taken at this point using a syringe or a phlebotomy adaptor, temporarily reattaching the tourniquet to generate adequate flow. The bung or IV administration set can then screwed in place over the hub, and the cannula should be secured to the skin with an appropriate dressing. A saline flush or a prepared bag of IV fluid with an administration set should be used to confirm adequate flow, observing for the absence of swelling or edema around the insertion site.
Various strategies have been suggested to increase the success of line placement, particularly in challenging circumstances, with varying degrees of evidence behind them.[4] Examples include:
- Gently ballotting or tapping the skin overlying the vein, and wiping the area with an antiseptic swab.
- Applying a warm compress, or soaking the limb in warm water, for a short period before line placement.
- Optimizing ergonomics for the operator placing the line, including patient position and ambient lighting.
- Apply topical aliquots of glyceryl trinitrate.
Where other attempts at peripheral access have failed in a critically ill patient requiring emergency intravenous access, peripheral venous cut-down may be considered. In this technique, a skin incision is made over a suitable peripheral site such as the median basilic vein in the arm or long saphenous vein in the leg, and the operator bluntly dissects down through the tissue to the vein, allowing insertion under direct vision.[4] Peripheral venous cut-down was once a mainstay of resuscitation, but with the advent of other modalities such as ultrasound-guidance, central venous access using the Seldinger technique, and intraosseous access, its importance has lessened and it is no longer common in many countries with well-resourced healthcare systems.[9]
Complications
Local complications of line placement include failure of the procedure, damage to arteries or nerves, and hematoma or bleeding at the insertion site. Of those IV lines successfully placed, up to 50% may develop some failure before their use is no longer clinically indicated.[10] Inadvertent arterial cannulation is more likely in children at specific sites such as the antecubital fossa,[11] and can pose serious consequences with the injection of incompatible medications or fluids. If this occurs, the line should be removed promptly with pressure applied until bleeding has ceased. Infiltration of the IV therapy into surrounding tissues may occur if the catheter migrates out of the vein over time, was incompletely threaded into the vein at the time of insertion, or is passed through the vein and out the far side. Phlebitis, or inflammation of the vein, is more likely where poor asepsis is observed and with increasing duration of IV therapy; it can progress to local infection or cellulitis. The catheter or vein may become occluded due to mechanical trauma, proximity to a valve within the vein, or thrombosis within the catheter tip. If an empty IV fluid administration set is left attached to the cannula, and especially if the limb is constricted (such as by non-invasive blood pressure monitoring or patient position), blood may overcome the residual pressure within the set and flow back into the line, leading to clotting and occlusion. Scalp lines in neonates and infants are associated with increased risk of dislodgement and extravasation.
Systemic complications, such as anaphylaxis, due to the procedure of line placement itself are rare, and allergic reactions may be more commonly attributable to medications or fluids administered through the line. Vasovagal syncope is more likely in patients who are sitting, rather than in the recumbent position, at the time of line placement; in those who have a history of fainting; or in those who have significant anxiety over the sight of blood or needles.[12][13] Part of the catheter may shear off inside the patient and embolize within the venous system which may necessitate retrieval by a vascular surgeon.[14]
Clinical Significance
Peripheral IV lines are the principal modality for the delivery of intravenous therapy in acute healthcare. Placement of peripheral lines is a core skill for many healthcare professionals and may also be performed by technicians or assistants depending on local practice and training.
According to the Hagen-Poiseuille equation (which describes flow dynamics for fluids undergoing fully developed laminar flow), the rate of flow through a cylindrical tube is directly proportional to the pressure gradient applied across the tube and inversely proportional to its length. However, flow is proportional to the fourth power of the internal radius, making this the single most important determinant of flow rates, and meaning that increasing the caliber of a line will have significantly more impact upon the potential maximum flow than reducing its length. A wide, short peripheral line may, therefore, achieve much higher flow rates than more technically advanced vascular access devices like central venous catheters.
Catheter diameter is measured using either the Birmingham gauge system or the French system. In the gauge system, a lower gauge number signifies a larger diameter. Peripheral IV lines are commonly available in sizes ranging from 14 to 26 gauge; 14 gauge is the widest of these, allowing for the highest potential flow rates. Nevertheless, persistent high flow rates may dramatically increase shear stress at the venous wall, which can result in endothelial dysfunction that precipitates earlier failure of the peripheral line.[15] The French system is based on the outer diameter of the catheter measured in millimeters, which is then multiplied by three, and therefore a catheter with an outer diameter of six millimeters corresponds to an 18 French catheter. In practice, the French system is not used for IV lines, and it is used for sizing larger medical devices and catheters designed for other purposes.
Enhancing Healthcare Team Outcomes
Optimal outcomes in IV therapy require an interprofessional team approach (primarily nurses and phlebotomy technologists reporting to managing physicians), with prompt placement when required, regular monitoring of line function, considering the ongoing need for venous access, removing lines when their presence is no longer clinically indicated, and early intervention if complications are suspected.[16]
Some healthcare facilities traditionally mandated that IV lines should be removed and replaced as a matter of practice after a certain period, such as 48 or 72 hours, to reduce the risk of complications. A 2018 systematic review of the literature with meta-analysis found no evidence that routine replacement of IV lines reduces the incidence of thrombophlebitis, catheter-related bloodstream infections, pain, or mortality (although it likely reduces rates of catheter blockage), and such practice may increase overall healthcare costs associated with line placement.[17]
The benefit of safety lines compared to standard lines is unclear. Users feel themselves to be at less risk of needlestick injury when using safety lines, but they are more expensive, may be more difficult to insert (partly due to slower flashback), and may increase the risk of blood splatter when the mechanism activates.[8][18] They may make line placement more difficult, due to slower flashback on venepuncture, greater friction when the cannula is advanced, and more difficulty in advancing the line into the vein.[19][7][19] A review in 2012 found that active safety lines were associated with increased rates of environmental contamination with blood, but this was not the case with passive safety lines. It did not find evidence that the use of safety lines is associated with a reduced likelihood of sharps injuries.[8]