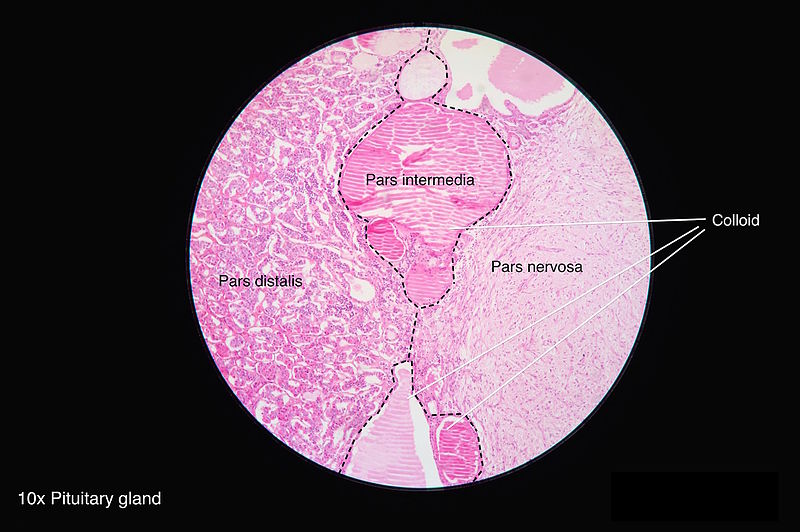
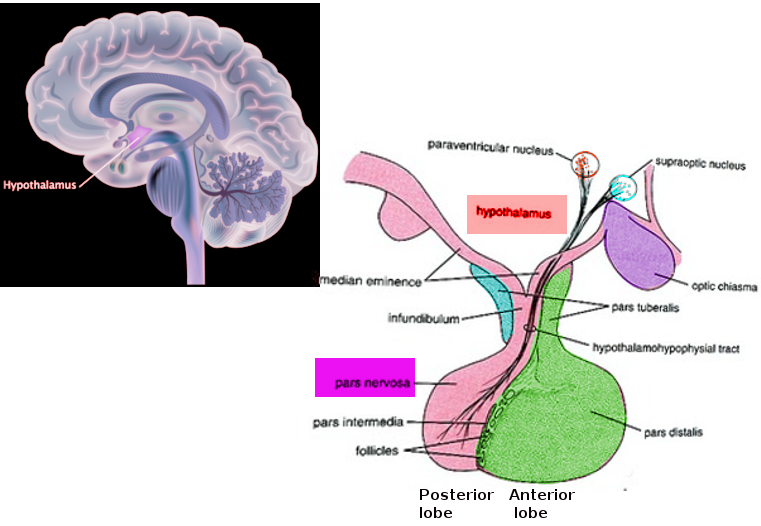
[1]
Amar AP, Weiss MH. Pituitary anatomy and physiology. Neurosurgery clinics of North America. 2003 Jan:14(1):11-23, v
[PubMed PMID: 12690976]
[2]
Thibonnier M, Preston JA, Dulin N, Wilkins PL, Berti-Mattera LN, Mattera R. The human V3 pituitary vasopressin receptor: ligand binding profile and density-dependent signaling pathways. Endocrinology. 1997 Oct:138(10):4109-22
[PubMed PMID: 9322919]
[3]
Musumeci G, Castorina S, Castrogiovanni P, Loreto C, Leonardi R, Aiello FC, Magro G, Imbesi R. A journey through the pituitary gland: Development, structure and function, with emphasis on embryo-foetal and later development. Acta histochemica. 2015 May-Jun:117(4-5):355-66. doi: 10.1016/j.acthis.2015.02.008. Epub 2015 Apr 6
[PubMed PMID: 25858531]
[4]
Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, Larkin S, Ansorge O. Development And Microscopic Anatomy Of The Pituitary Gland. Endotext. 2000:():
[PubMed PMID: 28402619]
[5]
Liu W, Xu ZM, Liu XM, Kong L, Yin WN, Wang GH. Microsurgical anatomy of perforated arteries in the hypothalamic area. Turkish neurosurgery. 2015:25(1):63-8. doi: 10.5137/1019-5149.JTN.9840-13.1. Epub
[PubMed PMID: 25640547]
[6]
Truong HQ, Najera E, Zanabria-Ortiz R, Celtikci E, Sun X, Borghei-Razavi H, Gardner PA, Fernandez-Miranda JC. Surgical anatomy of the superior hypophyseal artery and its relevance for endoscopic endonasal surgery. Journal of neurosurgery. 2018 Jul 13:131(1):154-162. doi: 10.3171/2018.2.JNS172959. Epub
[PubMed PMID: 30004277]
[7]
Auer MK, Stieg MR, Crispin A, Sievers C, Stalla GK, Kopczak A. Primary Empty Sella Syndrome and the Prevalence of Hormonal Dysregulation. Deutsches Arzteblatt international. 2018 Feb 16:115(7):99-105. doi: 10.3238/arztebl.2018.0099. Epub
[PubMed PMID: 29510819]
[8]
Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, Evanson J. Radiology of the Pituitary. Endotext. 2000:():
[PubMed PMID: 25905384]
[9]
Guerrero-Pérez F, Vidal N, Marengo AP, Pozo CD, Blanco C, Rivero-Celada D, Díez JJ, Iglesias P, Picó A, Villabona C. Posterior pituitary tumours: the spectrum of a unique entity. A clinical and histological study of a large case series. Endocrine. 2019 Jan:63(1):36-43. doi: 10.1007/s12020-018-1774-2. Epub 2018 Oct 1
[PubMed PMID: 30276594]
Level 2 (mid-level) evidence
[11]
Miller BA, Ioachimescu AG, Oyesiku NM. Contemporary indications for transsphenoidal pituitary surgery. World neurosurgery. 2014 Dec:82(6 Suppl):S147-51. doi: 10.1016/j.wneu.2014.07.037. Epub
[PubMed PMID: 25496626]
[12]
Asemota AO, Ishii M, Brem H, Gallia GL. Comparison of Complications, Trends, and Costs in Endoscopic vs Microscopic Pituitary Surgery: Analysis From a US Health Claims Database. Neurosurgery. 2017 Sep 1:81(3):458-472. doi: 10.1093/neuros/nyx350. Epub
[PubMed PMID: 28859453]
[13]
Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, Christ-Crain M, Ball S. The Neurohypophysis: Endocrinology of Vasopressin and Oxytocin. Endotext. 2000:():
[PubMed PMID: 25905380]
[14]
Di Iorgi N, Napoli F, Allegri AE, Olivieri I, Bertelli E, Gallizia A, Rossi A, Maghnie M. Diabetes insipidus--diagnosis and management. Hormone research in paediatrics. 2012:77(2):69-84. doi: 10.1159/000336333. Epub 2012 Mar 16
[PubMed PMID: 22433947]
[16]
Cuesta M, Thompson CJ. The syndrome of inappropriate antidiuresis (SIAD). Best practice & research. Clinical endocrinology & metabolism. 2016 Mar:30(2):175-87. doi: 10.1016/j.beem.2016.02.009. Epub 2016 Feb 27
[PubMed PMID: 27156757]