Introduction
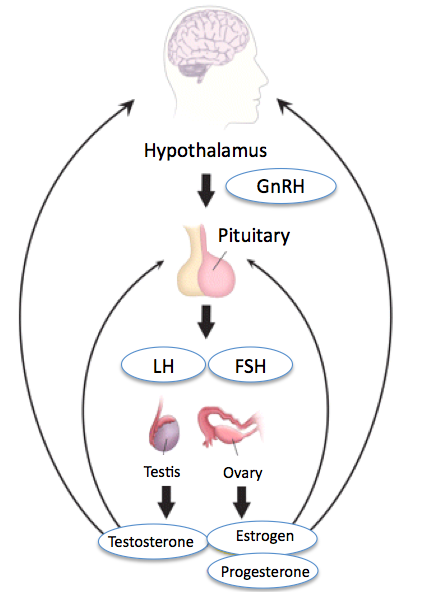
Luteinizing hormone (LH) is a glycoprotein hormone that is co-secreted along with follicle-stimulating hormone by the gonadotrophin cells in the adenohypophysis (anterior pituitary). Luteinizing hormone is a part of a neurological pathway comprised of the hypothalamus, the pituitary gland, and the gonads (see Image. Hypothalamic-Pituitary-Gonadal Axis). In this pathway, LH release is stimulated by gonadotropin-releasing hormone (GnRH) and inhibited by estrogen in females and testosterone in males. LH has various functions, which differ between women and men. In both sexes, LH contributes to the maturation of primordial germ cells. In men, LH causes the testes' Leydig cells to produce testosterone. In women, LH triggers the creation of steroid hormones from the ovaries.[1] Additionally, LH helps regulate the length and order of the menstrual cycle in females by playing roles in ovulation and implantation of an egg in the uterus.[2]
Cellular Level
Gonadotroph cells in the anterior pituitary gland produce luteinizing hormone. They have large, round cell bodies with prominent Golgi apparatus and endoplasmic reticulum. These cells are diffusely spread out and comprise 10% to 15% of the functional anterior pituitary cell mass. They do not react well with acid or basic stains and appear either basophilic or chromophobic under the microscope.[3]
LH and FSH are made from similar genes and thus have similar properties; they are both glycoproteins comprising alpha and beta subunits. The alpha subunit is the same between the 2 hormones, and the beta subunit of each is different and gives each hormone its biological specificity.[4] Specifically, the alpha subunit of LH is made up of 92 amino acids, and the beta subunit is made up of 120 amino acids. Combined, these 2 subunits have a mass of 28 kDa.[5]
Development
Fetal Development
LH and human chorionic gonadotropin (hCG) are 2 essential hormones in the development of both sexes. Their levels can be seen to fluctuate throughout development. In male fetuses, hCG begins at a high level in the plasma and quickly decreases between weeks 10 and 20 of gestation and then slowly declines afterward. In contrast, LH secretion increases by week 10, peaks before week 20, and decreases gradually. Increased plasma levels of HCG early on in gestation are a more significant contributor to testosterone production by Leydig cells than LH early in the development of a fetus. However, as LH levels rise, the regulation of testosterone formation changes to LH, which is driven by weeks 15 to 20 of gestation. This change in regulation can be exemplified by anencephalic male fetuses that are deficient in LH. In these fetuses, normal development of the male reproductive tract occurs while hCG levels are high initially. However, due to the lack of LH, the development of the external genitalia is impeded when hCG levels decrease around gestational weeks 15 to 20.[6]
In female fetuses, the peak levels of LH are higher than in male fetuses; this has been thought to be due to negative feedback of higher testosterone levels on the hypothalamic-pituitary-gonadal axis in male fetuses. Female fetuses have a lower level of gonadal hormones during gestation because the development of the female reproductive tract is not dependent on circulating levels of LH or hCG. The developing ovary does not express LH/choriogonadotropin receptors until the 16th week of gestation, which is why there is minimal steroidogenesis in the ovary until after delivery of the fetus.[6]
After Delivery
After delivery, regardless of sex, a sharp increase in LH levels is seen because the mother withdraws estrogen. After this temporary increase, LH levels begin to decline and stay at low basal levels until prepuberty starts in both sexes.[6]
Puberty
In the years leading up to puberty in both sexes, there is a slow increase in the secretion of LH nocturnally. As puberty progresses, LH begins to be secreted less so in a nocturnal pattern followed by a pulsatile pattern throughout the whole day. This increase in gonadotropin secretion helps to stimulate gonadal steroidogenesis, which is important for maturation.[6]
Organ Systems Involved
The primary organ systems in which LH is involved are the central nervous system (hypothalamus and pituitary) and the reproductive organ systems of both males and females. The hypothalamus secretes GnRH in a pulsatile manner, stimulating LH secretion. GnRH itself undergoes regulation by multiple neurotransmitters like dopamine, serotonin, norepinephrine, glutamate, opiate, and galanin. Kisspeptin is a crucial regulator of GnRH; it is an important GnRH secretagogue encoded by the KISS1 gene. Gonadal steroids, estrogen, progesterone, and testosterone exert negative feedback, thus decreasing the secretion of LH.[7]
Function
In males, LH stimulates testosterone release by the Leydig cells of the testes. In females, LH stimulates steroid release from the ovaries, ovulation, and the release of progesterone after ovulation by the corpus luteum.[8]
Ovulation
Ovulation is made possible by the combined actions of the hypothalamus, pituitary, and ovary. The hypothalamus begins the ovulation process by releasing GnRH in a pulsatile fashion. This pulsatile release causes the anterior pituitary to release LH and FSH, which then act on the ovarian follicle. This follicle comprises 3 essential cells: theca, granulosa, and oocyte. LH causes the theca cells to make androstenedione. Androstenedione then converts to estradiol via aromatase, which FSH stimulates. Upon achieving a critical concentration of estradiol, the negative feedback on LH that normally occurs by estrogen is shut off, and it begins to have positive feedback on LH release, which causes an "LH surge," which initiates ovulation. Once ovulation has occurred, the follicle becomes the corpus luteum. The corpus luteum secretes progesterone and is stimulated by LH or hCG if a pregnancy occurs.[9]
Mechanism
LH binds to a G-protein–coupled receptor, activating adenylyl cyclase. Adenylyl cyclase, an enzyme, then produces cyclic-AMP, thus increasing its intracellular concentration and activating a kinase molecule called protein kinase A (PKA). PKA then phosphorylates specific intracellular proteins that subsequently achieve the end physiological actions of LH, like steroid production and ovulation.[8]
Related Testing
Women use ovulation predictor kits to determine the exact time of ovulation while trying to get pregnant. These kits quantify LH levels in the urine.[10]
Clinical Significance
Testicular Dysfunction in Chronic Kidney Disease
Low libido, potency, and testicular size are all signs of testicular dysfunction. All these signs can be present in end-stage renal disease. Testosterone concentration in the plasma and how quickly testosterone production takes place are usually low in patients with chronic kidney disease. Spermatogenesis has been noted to be either lowered or completely absent as well. After renal transplantation, the abovementioned changes can be reversed and return to normal. Studies have shown that this testicular dysfunction and altered testosterone concentration results from higher levels of LH in the plasma and lower amounts of secretory LH pulses seen in men with end-stage renal disease when compared to healthy subjects or men who underwent a successful renal transplant. This is significant because LH's pulsatile secretion is necessary for the testes' gonadotropin receptors to function properly. Furthermore, sustained high levels of LH in the blood and testes can cause a loss of gonadotropin receptors in the testes.[11]
Infertility and Assisted Reproductive Technology
Infertility is defined clinically as the inability to become clinically pregnant after at least 12 months of unprotected sexual intercourse. Female factors, male factors, or both can cause it. In women, it can be the result of ovulatory issues (ie, anovulation), obstructions of the fallopian tubes, and endometriosis. To become pregnant, many women undergo assisted reproductive technologies (ARTs), like intrauterine insemination and in vitro fertilization.[12]
Proper development of a follicle and ovulation involves the combined effects of follicle-stimulating hormone (FSH) and LH and their bodily activities. This interplay between FSH and LH has also been shown to be important in ART. It has been found that low LH levels in the body can result in poor outcomes in ART. Thus, patients who have low endogenous LH, such as those with hypogonadotropic hypogonadism, can have an increase in the efficacy of ART with exogenous LH treatment.[13] A further study found that with supplementation of LH during the mid-follicular phase, there were better pregnancy results in women who had not responded optimally to conventional ART. This outcome was thought to be due to the increased production of 17-beta-estradiol.[2] Another study reported that fertilization, implantation, and clinical pregnancy rates were higher in patients who underwent ART that included recombinant human LH (r-hLH) when compared to patients who underwent ART without r-hLH. A lower apoptosis rate was also present in patients who underwent ART, which included r-hLH.[5]
Although there are demonstrable benefits of LH supplementation during ART, research also shows that LH levels can have unfavorable effects on ART.[13] These adverse effects are thought to result from inhibition of granulosa cell proliferation, atresia of immature follicles, and luteinization of preovulatory follicles before they would be under physiological conditions. Additionally, increased LH before ovulation has been shown to influence the conception and implantation of the embryo negatively.[2]
Hypogonadotropic Hypogonadism in Males
Hypogonadism is impaired testicular function; this can occur due to a problem with the testes (primary or hypogonadotropic hypogonadism) or due to a problem with the hypothalamic-pituitary-gonadal axis (secondary or hypogonadotropic hypogonadism). Men with hypogonadotropic hypogonadism have low levels of androgens in the plasma as well as a lack or delay of sexual maturity, which can cause symptoms such as a lack of libido, depression, increase in adipose tissue, and diminished erectile function.[14]
Patients with hypogonadotropic hypogonadism usually have an issue with GnRH signaling, which then causes a decrease in FSH and LH secretion. This decreased FSH and LH contributes to decreased androgen levels and reduced spermatogenesis. Studies have shown that giving these patients pulsatile GnRH or LH (or hCG) and FSH can help increase spermatogenesis and thus increase the sperm concentration in the ejaculate. Even then, most couples will need ART to achieve pregnancy.[15]