Introduction
Corticobulbar tract supplies upper motor neuron innervation to the cranial nerves supplying head and face. The precentral gyrus in the posterior part of the frontal lobe of the cerebrum is the part of the primary motor cortex from where several motor pathways originate. Some fibers to cortico-bulbar tract (as with corticospinal tract) also come from the Pre-motor area and supplementary area.
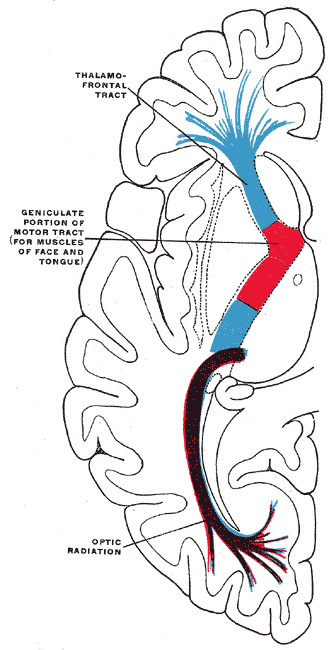
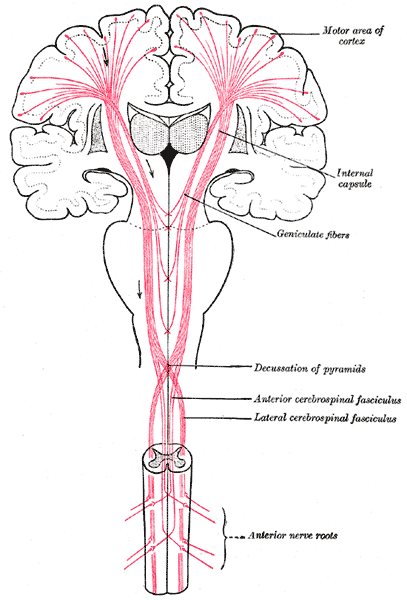
The corticobulbar tract starts in the precentral gyrus. From here, the corticobulbar fibers pass through the corona radiata, genu of the internal capsule, peduncle of the cerebrum, base of the pons, and then to the medullary pyramid. They synapse at the motor nuclei of cranial nerve nuclei. The corticobulbar tract may synapse either directly on the motor nuclei or indirectly through interneurons. Corticobulbar tract supplies input bilaterally to trigeminal, facial, and hypoglossal nerve nuclei.[1]
Structure and Function
Cell bodies of these neurons are situated mainly in the Broadman’s areas number 4, 6. The organization of the motor cortex is in a somatotopic manner. Cortical homunculus is a map of the representation of different parts of the body in specific areas of the motor cortex with face situated most laterally.
From the cerebral cortex, the corticobulbar fiber passes into the corona radiata. Most of the fibers of corona radiata arrange themselves into a compact bundle called the internal capsule, which is situated between caudate nucleus and thalamus medially and lentiform nucleus laterally. Corticobulbar fibers pass through the genu or bend of the internal capsule. From the internal capsule, these fibers enter into crus cerebri of the cerebral peduncles in the midbrain. Corticobulbar fibers pass through middle 1/3 of crus cerebri and then enter the pons and medulla to terminate at corresponding nerve nuclei.
The corticobulbar tract provides input to the nuclei innervating muscles of the face, muscles of mastication, muscles of the tongue, muscles of the pharynx, muscles of larynx, sternocleidomastoid, and trapezius, etc. This innervation is bilateral for most of the cranial nerves, with the exception of corticobulbar fibers providing input to the lower facial nucleus, which receives contralateral innervation. Both right and left half of the motor cortex of the brain gives fibers to the dorsal division of the motor nucleus of the facial nerve ( bilateral innervation), but the lower division of facial nerve nucleus receives unilateral upper motor neuron fibers. The dorsal division of the facial nerve nucleus gives lower motor neuron fibers to the upper half of the face, and the lower half of the face receives fibers from the ventral division of the facial nerve nucleus.[1][2][3][4]
Embryology
The cerebral hemisphere forms from the telencephalon. The cerebral cortex forms from the roof and lateral aspect of telencephalon forming cerebral hemisphere. Initially, no sulci, gyri are present, but after six months of intrauterine life, sulci and gyri start appearing. The precentral gyrus forms in front of central sulcus. Cranial nerve motor nuclei formed from the basal plate of the brain stem. Processes of neurons situated in percental gyrus terminating at cranial nerve nuclei directly or indirectly through interneuron form corticobulbar tract.[5]
Blood Supply and Lymphatics
Corticobulbar tact receives supply from branches of the middle cerebral artery, anterior cerebral artery, and basilar artery.[6]
Thrombosis, embolism, rupture, trauma, or compression of a blood vessel supplying genu of the internal capsule can cause injury to corticobulbar and adjacent tracts. As in the internal capsule, tracts are closely packed, infarction of small areas can lead to damage of several descending and ascending tracts.
Muscles
Corticobulbar tract carries upper motor neuron input to motor nuclei of trigeminal, facial, glossopharyngeal, vagus, accessory, and hypoglossal nerves. The motor component of trigeminal nerves supplies muscles of mastication. The facial nerve supplies the muscles of facial expression. Glossopharyngeal, vagus, accessory nerve innervates muscles of the pharynx, muscles of larynx, sternocleidomastoid, and trapezius muscle. Tongue muscles receive fibers from the hypoglossal nerve.
The corticobulbar tract indirectly affects muscles supplied by third, fourth, and sixth cranial nerve through medial longitudinal fasciculus and paramedian part of the pontine formation.
Physiologic Variants
The corticobulbar tract extends from the precentral cortex to cranial nerve nuclei supplying the head and neck region. Similarly, upper motor fibers for the body are carried by the corticospinal tract, which connects the cerebral cortex to the spinal cord.[3]
Surgical Considerations
Injury to corticobulbar tract between the cerebral cortex and brain stem leads to symptoms of upper motor neuron damage of cranial nerves supplying the face, head, and neck. Common causes of damage to the corticobulbar tract are cerebrovascular accidents, space-occupying lesions, trauma, degenerative diseases of the brain, etc. Symptoms of corticobulbar fiber lesion are dysarthria, dysphagia, difficulty in the jaw and facial movements, spasm of the tongue, and increased reflex.[7][8]
Clinical Significance
Pseudobulbar palsy is a condition in which there is bilateral damage to corticobulbar fibers leading to dysarthria, dysphagia, spasticity of the tongue, hyperreflexia, and difficulty with jaw and facial movements. The pseudobulbar impact of the labile effect is a common symptom of pseudobulbar palsy. The pseudobulbar affect or labile effect characteristically presents as an episode of emotional imbalance (excessive crying or laughing).[9][10]
Pseudobulbar palsy can be due to multiple reasons such as:
- Infarction of cerebral hemisphere bilaterally
- Demyelinating motor neuron disease
- Tumors in the upper part of the brain stem
- Amyotrophic lateral sclerosis
- Parkinson’s Disease
- Multiple sclerosis
- Trauma
If there is damage to the corticobulbar tract of one side anywhere between precentral gyrus to the motor nucleus of the facial nerve. It results in paralysis of muscles of the opposite lower half of the face.
For example- If a corticobulbar tract of right side carrying fibers to the facial nucleus gets damaged, facial muscles of the lower half of the face on the left side get paralyzed. Muscles of the upper half of the left side remain unaffected as it receives innervation from corticobulbar fibers of facial nerve of both sides.
Supranuclear lesions of the hypoglossal nerve can occur at any site extending from the cerebral cortex to hypoglossal nerve nuclei. Tongue atrophy does not occur in supranuclear lesions, but it can lead to uncoordinated, slow but spastic tongue movements. Infranuclear lesions of hypoglossal nerve cause weakness, ipsilateral atrophy of the tongue with fasciculations.
Amyotrophic lateral sclerosis is a disease in which there is the progressive degeneration of neuronal tissue. When degeneration involves corticobulbar tract signs and symptoms of upper motor neuron disease in the form of muscular weakness, difficulty in speech, difficulty in tongue movement appears.