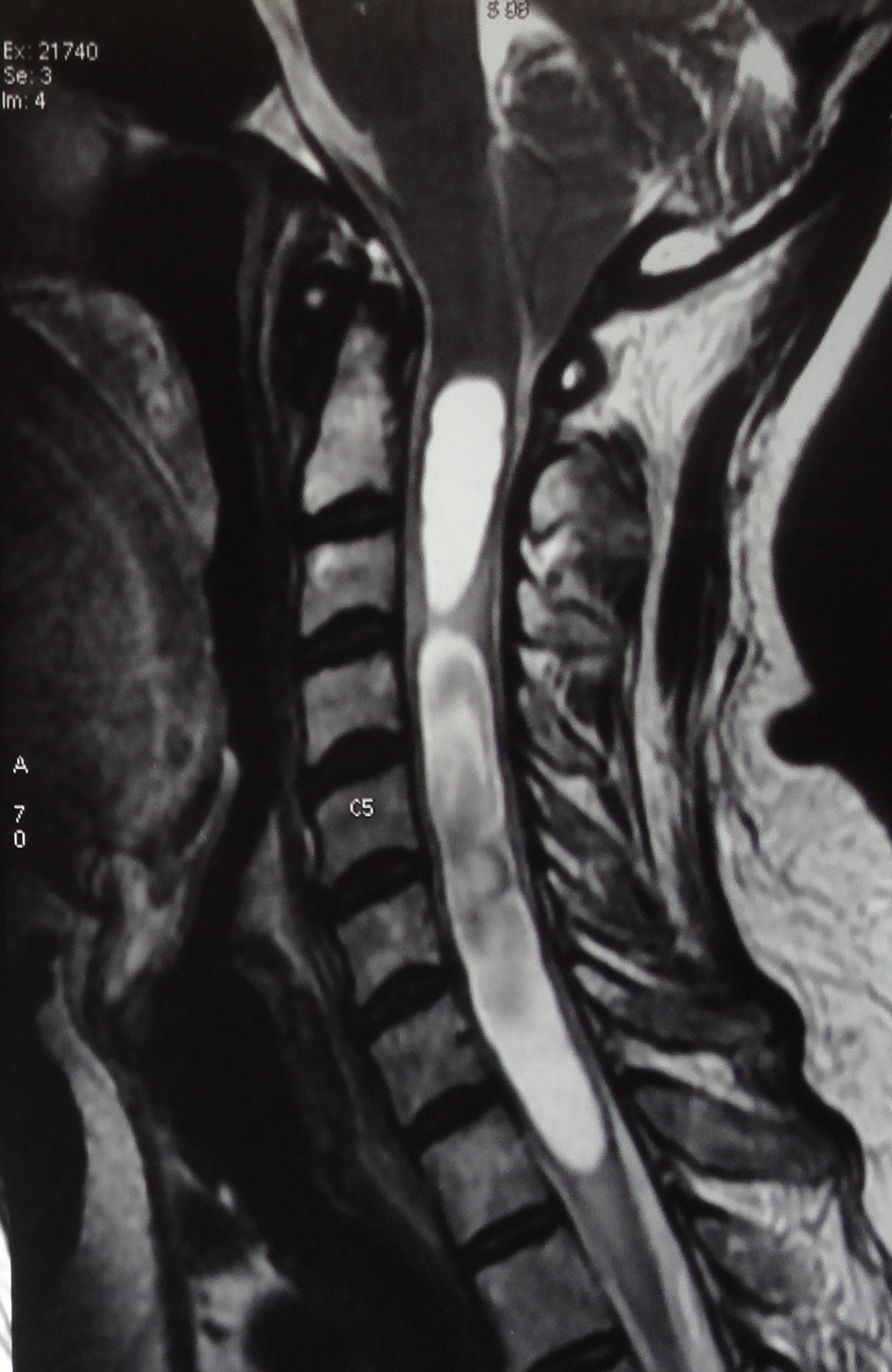
[1]
Krebs J, Koch HG, Hartmann K, Frotzler A. The characteristics of posttraumatic syringomyelia. Spinal cord. 2016 Jun:54(6):463-6. doi: 10.1038/sc.2015.218. Epub 2015 Dec 1
[PubMed PMID: 26620880]
[2]
Bonfield CM, Levi AD, Arnold PM, Okonkwo DO. Surgical management of post-traumatic syringomyelia. Spine. 2010 Oct 1:35(21 Suppl):S245-58. doi: 10.1097/BRS.0b013e3181f32e9c. Epub
[PubMed PMID: 20881468]
[3]
Schurch B, Wichmann W, Rossier AB. Post-traumatic syringomyelia (cystic myelopathy): a prospective study of 449 patients with spinal cord injury. Journal of neurology, neurosurgery, and psychiatry. 1996 Jan:60(1):61-7
[PubMed PMID: 8558154]
[4]
Karam Y,Hitchon PW,Mhanna NE,He W,Noeller J, Post-traumatic syringomyelia: outcome predictors. Clinical neurology and neurosurgery. 2014 Sep
[PubMed PMID: 25016238]
[5]
Lam S, Batzdorf U, Bergsneider M. Thecal shunt placement for treatment of obstructive primary syringomyelia. Journal of neurosurgery. Spine. 2008 Dec:9(6):581-8. doi: 10.3171/SPI.2008.10.08638. Epub
[PubMed PMID: 19035753]
[6]
Kim HG, Oh HS, Kim TW, Park KH. Clinical Features of Post-Traumatic Syringomyelia. Korean journal of neurotrauma. 2014 Oct:10(2):66-9. doi: 10.13004/kjnt.2014.10.2.66. Epub 2014 Oct 31
[PubMed PMID: 27169036]
[7]
Goldstein B, Hammond MC, Stiens SA, Little JW. Posttraumatic syringomyelia: profound neuronal loss, yet preserved function. Archives of physical medicine and rehabilitation. 1998 Jan:79(1):107-12
[PubMed PMID: 9440427]
[8]
Li YD, Therasse C, Kesavabhotla K, Lamano JB, Ganju A. Radiographic assessment of surgical treatment of post-traumatic syringomyelia. The journal of spinal cord medicine. 2021 Nov:44(6):861-869. doi: 10.1080/10790268.2020.1743086. Epub 2020 Mar 30
[PubMed PMID: 32223591]
[9]
Lee TT, Alameda GJ, Gromelski EB, Green BA. Outcome after surgical treatment of progressive posttraumatic cystic myelopathy. Journal of neurosurgery. 2000 Apr:92(2 Suppl):149-54
[PubMed PMID: 10763684]
[10]
Killeen T, Rosner J, Jutzeler CR, Hupp M, Heilbronner R, Curt A. Spontaneous resolution of an extensive posttraumatic syrinx. Neurology. 2016 Sep 20:87(12):1299-301. doi: 10.1212/WNL.0000000000003130. Epub 2016 Aug 19
[PubMed PMID: 27543642]
[11]
Maharaj MM, Phan K, Mobbs R. Spontaneous regression of post-traumatic syringomyelia: A case report and literature review. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2017 Oct:44():249-253. doi: 10.1016/j.jocn.2017.06.065. Epub 2017 Jul 14
[PubMed PMID: 28712735]
Level 3 (low-level) evidence
[12]
Srikantha U, Hari A, Lokanath YK, Varma RG. Syringo-Subarachnoid Shunt Placement: A Minimally Invasive Technique Using Fixed Tubular Retractors-Three Case Reports and Literature Review. International journal of spine surgery. 2020 Apr:14(2):133-139. doi: 10.14444/7020. Epub 2020 Apr 30
[PubMed PMID: 32355617]
Level 3 (low-level) evidence
[13]
Hussain I, Greenfield JP. Ultrasound-guided Syringosubarachnoid Shunt Insertion for Cervicothoracic Syringomyelia. Clinical spine surgery. 2020 Jun:33(5):185-191. doi: 10.1097/BSD.0000000000000835. Epub
[PubMed PMID: 31220040]
[14]
Aghakhani N, Baussart B, David P, Lacroix C, Benoudiba F, Tadie M, Parker F. Surgical treatment of posttraumatic syringomyelia. Neurosurgery. 2010 Jun:66(6):1120-7; discussion 1127. doi: 10.1227/01.NEU.0000369609.30695.AB. Epub
[PubMed PMID: 20495426]
[15]
Batzdorf U, Klekamp J, Johnson JP. A critical appraisal of syrinx cavity shunting procedures. Journal of neurosurgery. 1998 Sep:89(3):382-8
[PubMed PMID: 9724111]
[16]
Falci SP, Indeck C, Lammertse DP. Posttraumatic spinal cord tethering and syringomyelia: surgical treatment and long-term outcome. Journal of neurosurgery. Spine. 2009 Oct:11(4):445-60. doi: 10.3171/2009.4.SPINE09333. Epub
[PubMed PMID: 19929342]
[17]
Holly LT, Johnson JP, Masciopinto JE, Batzdorf U. Treatment of posttraumatic syringomyelia with extradural decompressive surgery. Neurosurgical focus. 2000 Mar 15:8(3):E8
[PubMed PMID: 16676931]
[18]
Klekamp J. Treatment of posttraumatic syringomyelia. Journal of neurosurgery. Spine. 2012 Sep:17(3):199-211. doi: 10.3171/2012.5.SPINE11904. Epub 2012 Jul 13
[PubMed PMID: 22794351]
[19]
Vaquero J, Hassan R, Fernández C, Rodríguez-Boto G, Zurita M. Cell Therapy as a New Approach to the Treatment of Posttraumatic Syringomyelia. World neurosurgery. 2017 Nov:107():1047.e5-1047.e8. doi: 10.1016/j.wneu.2017.08.019. Epub 2017 Aug 10
[PubMed PMID: 28804041]
[20]
Levi V, Franzini A, Di Cristofori A, Bertani G, Pluderi M. Subacute posttraumatic ascending myelopathy (SPAM): A potential complication of subarachnoid shunt for syringomyelia? The journal of spinal cord medicine. 2020 Sep:43(5):714-718. doi: 10.1080/10790268.2018.1512735. Epub 2018 Aug 29
[PubMed PMID: 30156977]
[21]
Caremel R, Hamel O, Gerardin E, Lenormand L, Parker F, Lefort M, Grise P, Perrouin-Verbe B. [Post-traumatic syringomyelia: What should know the urologist?]. Progres en urologie : journal de l'Association francaise d'urologie et de la Societe francaise d'urologie. 2013 Jan:23(1):8-14. doi: 10.1016/j.purol.2012.09.009. Epub 2012 Oct 15
[PubMed PMID: 23287478]