Introduction
The auditory system functions to collect sound waves from the environment, transform mechanical vibrations from those sound waves into electrical nerve signals, which can be relayed to various areas of the central nervous system, and process sound into meaningful content. The complexity of auditory processing is apparent by the system's ability to localize, analyze, and interpret a sound that then extrapolates into useful information that the individual can respond to with simultaneous integration with other sensory stimuli. For a sound to be perceived by the individual, it needs to travel to and be processed by higher-order regions in the cerebral cortex, specifically the primary auditory area. From there, through bottom-up and top-down signaling pathways, the information can be relayed to other areas of the central nervous system, cerebral cortex, and lower brainstem regions to make meaning of the information as well as integrate auditory and other sensory stimuli. The primary auditory area acts as the principal location that receives sounds from peripheral auditory structures and is integral to begin the process of complex sound interpretation as well as the conscious perception of noise.[1][2]
Structure and Function
Structure of the Primary Auditory Cortex:
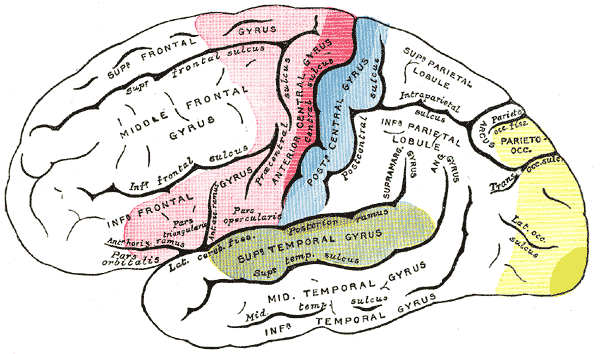
The primary auditory areas are regions of the cerebral cortex located bilaterally in the temporal lobes. The primary auditory area is housed within Heschl gyrus, a region that is positioned posteriorly in the superior temporal lobe within the supratemporal plane. This cortex, along with associated auditory areas, is clustered surrounding the posterior aspect of the Sylvian fissure or lateral sulcus of the cerebral cortex, which separates the temporal lobe inferiorly from the parietal and frontal lobe superiorly. Heschl gyrus cannot be visualized from a lateral view of the cerebral cortex since it is located deep to the superficial temporal lobe structure and is within the lateral sulcus. It runs towards the center of the brain in a medial-posterior fashion. The left Heschl gyrus in the majority of individuals is significantly longer when compared to the right gyrus, suggesting a correlation between left-hemisphere language dominance and associated differences in anatomical structure.[3][4]
Pathway of Sound from Peripheral to Central Auditory Structures:
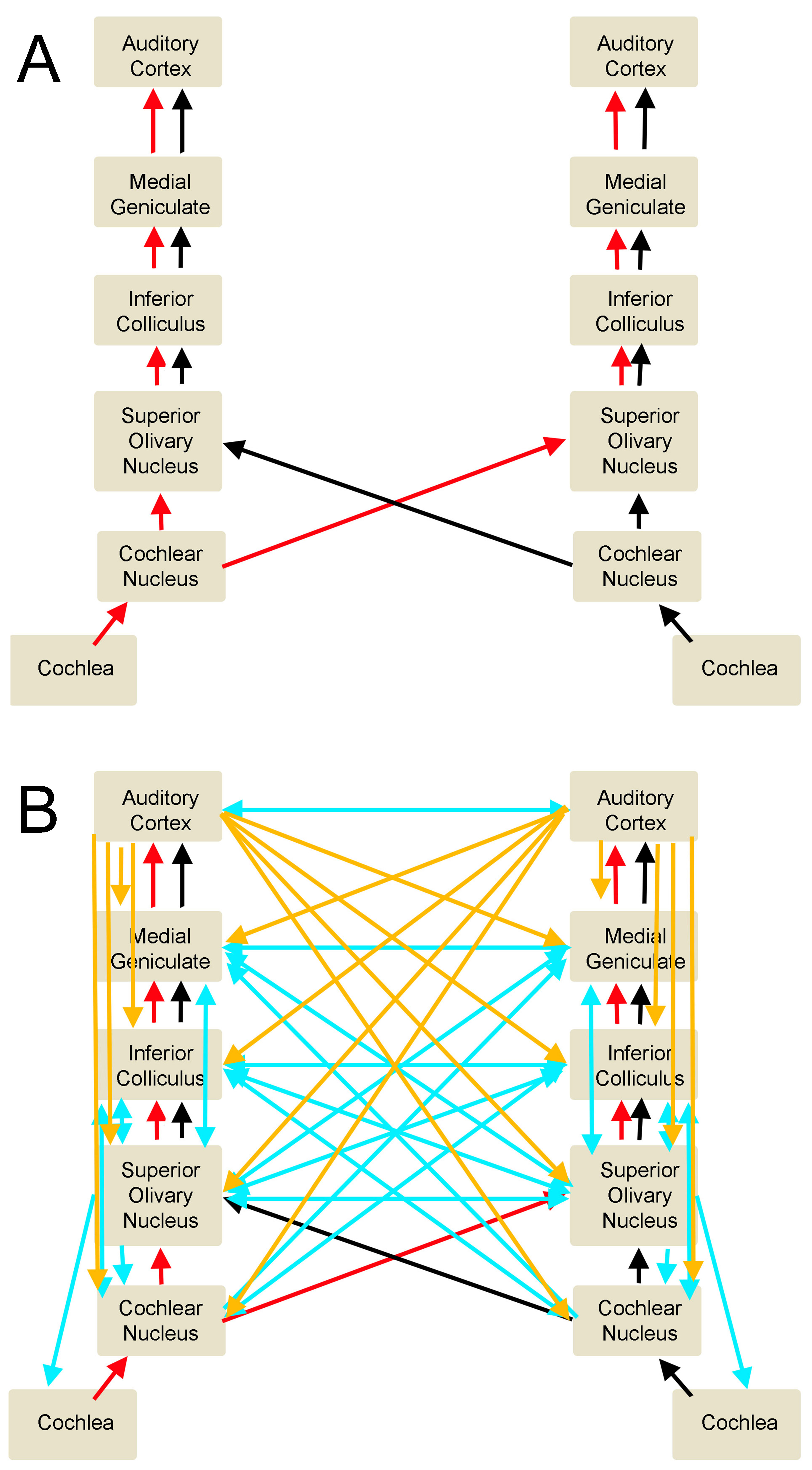
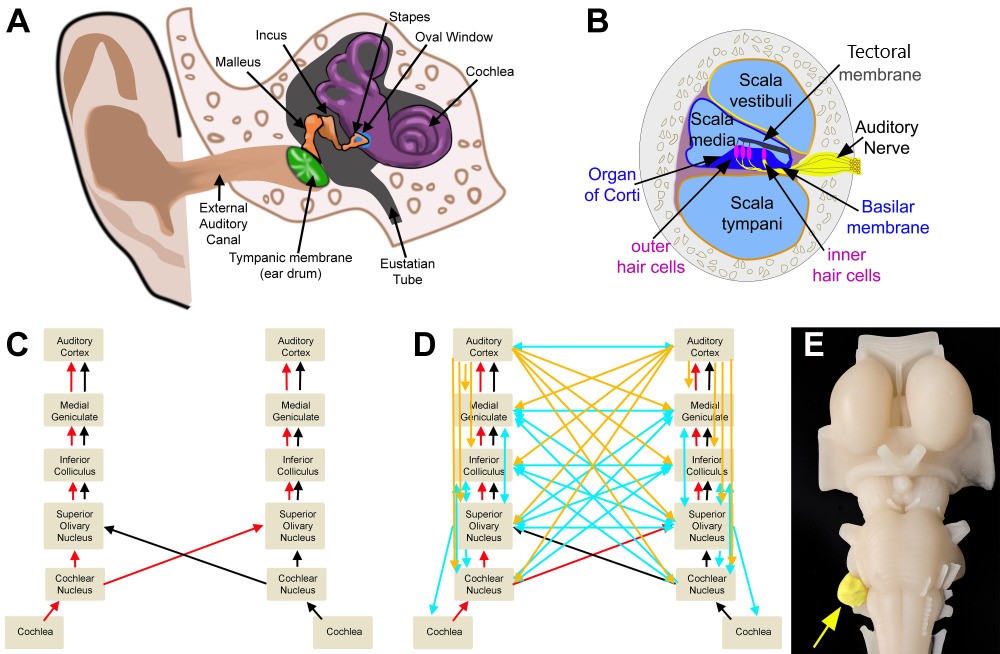
The path sound takes from the external environment to the primary auditory area and associated auditory areas, is complicated by many synapses, decussations, and inputs to brainstem nuclei bilaterally and both cerebral hemispheres. As sound waves travel through the air and are collected by the pinna of the ear, they are transmitted down the external auditory canal where they will produce vibrations of the tympanic membrane. This vibration of the tympanic membrane is translated to movement and vibration of three ossicles present in the middle ear, the malleus, incus, and stapes, to further transmit vibrations to the oval window of the inner ear. Vibrations then travel to the cochlea and are sensed by inner and outer hair cells of the organ of Corti, which function to transmit the mechanical energy present in vibrational sound waves into the electrical energy transmitted along the auditory nerve.[5]
The auditory nerve then transmits the signal to the cochlear nucleus located between the pons and the medulla in the brainstem. The signal then travels to the superior olivary nucleus in the pons, up through the lateral lemniscus pathway, then to the inferior colliculus of the midbrain, and onto the medial geniculate nucleus of the thalamus, and finally synapsing in the primary auditory cortex. During this travel, information decussates or crosses to the contralateral side of the brainstem. This crossing establishes both ipsilateral as well as contralateral input, with the majority of the fibers taking a contralateral pathway from each ear, which assists in localization and interpretation of sound quality. If the individual hears a sound from a location directly in the midline, the sound will reach both ears simultaneously. However, if sound production is from one side of a person’s midline, the sound will reach the closer ear before the other ear and will be of a louder intensity due to the individual’s head acting as an “acoustic shadow” to dampen the noise received by the far ear. In addition to ascending input pathways that the peripheral system provides to the primary auditory cortex, there are descending and output pathways that travel from the cerebral cortices down to brainstem nuclei. This top-down signaling pathway from the cerebral cortex allows for modulation of peripheral structures that respond to the attention of the individual as well as the relevance of the auditory stimulation that will dictate the individual’s behavioral response to that sound.[5][6][7][8][9][10][11][12][13][14]
The Primary Auditory Cortex and Associated Auditory Areas:
The primary auditory cortex can further subdivide into different regions based on structural and functional properties. These regions differ based on their cytoarchitecture, the number, organization, and type of neuron, myeloarchitecture, the amount and arrangement of myelinated fibers going to and coming from the cortex, as well as the chemoarchitecture, which is the differences in neurotransmitters and proteins expressed in that region of the brain. The structure of the primary auditory cortex is composed of a central core region (area 41), which is surrounded by a belt region that is subsequently surrounded by a parabelt region. Each of these regions differs based on their cellular architecture, response to stimuli, as well as their input and output pathways.[2]
The major inputs to the core area of the primary auditory cortex are the various regions of the medial geniculate nucleus of the thalamus, respectively, the ventral, dorsal, and the magnocellular components. The associated connections of both the inputs and outputs of the auditory cortical area are structured in a serial and parallel fashion. Serial connections proceed from the core region to the belt region, to finally the parabelt region. The belt and parabelt regions then form connections to various auditory associated regions in the cerebral cortex, namely regions surrounding the superior temporal sulcus. Parallel connections occur due to multiple outputs from each region of the medial geniculate body to more than one region of the auditory cortex. These various regions of the auditory cortex receive complex signals from multiple sources allowing them to integrate the information into meaningful information that can be relayed to other areas of the cerebral cortex.[3][2]
Once the information reaches the auditory cortex, more complex integration and interpretation of the stimulus can take place. Each primary auditory cortex has connections within the same cerebral hemisphere as well as between cerebral hemispheres. The main associated auditory areas that receive projections from the primary auditory cortex are regions of the superior temporal lobe surrounding Heschl gyrus, inferior parietal lobe, inferior-posterior frontal lobe, insula, amygdala, and basal ganglia. The destination of the outputs from the primary auditory cortex determines what higher-order and more complex cognitive functions take place with the incoming acoustic information. For example, auditory information traveling to the amygdala has profound impacts on the emotional and behavioral response of an individual, whereas information destined for the premotor cortex will be utilized for planning and controlling speech.[3][2]
Organization of the Auditory System:
The organization of the auditory system, and thus the corresponding function, depends on the hemisphere it is in, the location of the sound-sensitive neurons in that hemisphere, as well as what pathway through which the information is traveling. For example, acoustic stimulation in one ear produces a higher rate of cortical firing in the contralateral primary auditory cortex when compared to the ipsilateral cortex, suggesting a higher number of fibers crossing over in brainstem pathways. This observation has also been demonstrated in individuals with temporal lobe damage, including the primary auditory area and their associated difficulty localizing sound in their contralateral ear.[3] Two different pathways are known as major routes for sound information, and each carries its own type of sound information. The ventral stream carries semantic information, which is important for determining the meaning of language and travels from the rostral pole of the temporal lobe to the occipitotemporal cortex. The dorsal stream carries phonological information about sounds from the superior temporal cortex to the inferior frontal cortex, which aids the individual in understanding segments of speech, learning vocabulary, and understanding articulation of words.[15]
A common theme present in the organization of the auditory system is that the arrangement of neurons is in a tonotopic manner. This theme originates in the cochlea, with high frequencies located at the base of the cochlea closest to the oval window, and lower frequencies present at the apex of the cochlea.[5] This separation of sound frequencies and the established tonotopic gradient is maintained through each brainstem nuclei and is ultimately present in the primary auditory cortex. Each area is distinct in its function.[2] In the primary auditory area, the location of high frequencies is caudally and medially, while lower frequencies are rostrally and laterally.[3]
Embryology
Development of the central nervous system begins at gestational week three and continues throughout gestation and into postnatal life throughout adolescents and possibly throughout the lifespan. At the end of the third gestational week, the embryo forms a three-layered structure through the process of gastrulation and, shortly after, will undergo neurulation to form the neural plate and eventually, the neural tube at around embryonic days 20 through 27. Neural progenitor cells, which arise from the ectoderm, the outermost layer of the three-layered embryo, develop at around embryonic day 42.[16]
Just prior to the closure of the neural tube, approximately at days 25 to 27, the anterior region of the neural tube forms three pouches, also known as vesicles. These vesicles are the prosencephalon, the mesencephalon, and the rhombencephalon, from cranial to caudal in orientation. At approximately week 8 of gestation, at the end of the embryonic period of development, the three-vesicle stage transitions into a five-vesicle stage. The prosencephalon subdivides into the telencephalon and the diencephalon. The mesencephalon does not further divide. The rhombencephalon subdivides into the metencephalon and the myelencephalon. The telencephalon is destined to become regions of the forebrain and thus gives rise to the cerebral cortex. Since the primary auditory area is part of the temporal lobe of the cerebral cortex, its embryonic origin is the telencephalon.[16]
Full development of the cerebral cortex occurs during the postnatal period, where external environmental stimuli shape the developing brain by reinforcing synapses that are required for interaction with the environment, pruning unnecessary connections, and myelinating pathways to provide for faster signal conduction. These normal external stimuli are necessary for proper cortical development and organization, and deprivation of auditory sensory stimuli results in abnormalities in the organization and function of the primary auditory cortex.[16]
Blood Supply and Lymphatics
The major blood supply for the primary auditory cortex is the middle cerebral artery. The middle cerebral artery is a branch of the internal carotid artery and supplements the anterior cerebral artery as part of the anterior circulation of the brain. The most proximal segment of the middle cerebral artery (M1) runs lateral to the optic chiasm after branching from the internal carotid artery and travels through the lateral sulcus. The middle cerebral artery runs along the lateral sulcus in an anterior to posterior fashion to supply much of the lateral cerebral cortex and insula as part of the M2 region. The middle cerebral artery then, most commonly, bifurcates to form terminal branches that supply much of the cortex.[17]
The first branch that comes off of the middle cerebral artery to supply the auditory regions is the frontal-opercular artery and provides the primary blood supply to the anterior insula by running superior from its branch point. The next artery is the central sulcus artery, which supplies the posterior insula and much of the anterior parietal lobe. The following three arteries then branch inferiorly from the middle cerebral artery are the anterior, middle, and posterior temporal arteries and provide blood to the middle temporal gyrus and posterior temporal gyrus. Lastly, the angular artery, as well as parts of the middle cerebral artery itself, supply blood to the primary auditory area, the angular gyrus, and the supramarginal gyrus.[3]
Vascular injury, such as an ischemic or hemorrhagic stroke, is the most common form of insult to the primary auditory cortex. Cortical ischemia and necrosis that result due to a reduction in blood supply and nutrients to the middle cerebral artery or any of its branches can cause irreversible damage to the primary auditory cortex and associated auditory areas. The majority of input to the primary auditory cortex originates in the contralateral ear; one would expect vascular insults to have a greater auditory deficit in the contralateral ear when compared to the ipsilateral ear.
Physiologic Variants
There is considerable variation in the primary auditory area between individuals. There are also anatomical variations noted between hemispheres. Research has demonstrated that individuals who had the left Heschl gyrus stimulated during surgical procedures reported hearing voices and language; however, with stimulation of the right Heschl gyrus, the patients reported hearing music.[3] Similarly, professional musicians have larger densities and volumes of the right primary auditory cortex when compared to non-musicians. There have also been reported duplications of Heschl gyrus in some individuals, occurring on the left and right in equal frequencies, as well as bilaterally.[4]
Surgical Considerations
Due to the significant differences between individuals in the location of auditory-sensitive neurons and the various anatomical conformations of the primary auditory cortex, extensive pre-operative planning is necessary to reduce the possibility of damaging critical language and sound-sensitive areas. Functional mapping of the auditory cortex almost always needs to take place before surgical operations that could involve regions of the primary auditory cortex and is the gold standard pre-operative procedure. Functional MRI visualization of brain areas sensitive to auditory stimuli are not specific enough and cannot delineate clear margins necessary to determine which brain tissue is critical for sound and language functions and should only serve as an adjunctive method to visualize auditory-associated areas.[15]
Clinical Significance
Congenital Deafness:
Patients who are congenitally deaf never receive auditory stimulation to the primary auditory cortex. Even though these regions of the brain are not stimulated by sound information, studies have shown no difference in the overall volume of Heschl gyrus between patients who are congenitally deaf and hearing controlled patients. Patients who were congenitally deaf demonstrated that they had larger gray matter to white matter ratios in Heschl gyrus compared to hearing controls, demonstrating a reduction in afferent or efferent fibers and/or myelination originating from the primary auditory area. Research has shown that individuals who communicate using American Sign Language respond to visual stimuli of another individual signing with increased activity in the primary auditory cortex. This finding can be explained by neural plasticity, a phenomenon that explains that neurons can change their function and adapt to the surrounding environment based on stimuli that are received.[18]
Primary Progressive Aphasia:
Patients who have primary progressive aphasia have difficulties with speech production, understanding, and communication, often secondary to a neurodegenerative process such as Alzheimer Disease. Patients presenting with the non-fluent variant of primary progressive aphasia demonstrate increased effort associated with speaking, slowed speech production, difficulties utilizing grammar correctly, as well as reduced communication attempts, which can eventually lead to the patient becoming mute as their disease progresses. Studies have demonstrated that patients with non-fluent primary progressive aphasia have a reduction in the activity of the primary auditory area after auditory stimulation, suggesting that neurodegeneration is occurring in regions of Heschl gyrus.[19]
Mild Traumatic Brain Injury:
Individuals suffering from symptoms after a traumatic brain injury often report difficulties associated with auditory processing. Researchers have shown through fMRI studies that individuals with diagnosed mild traumatic brain injuries have less primary auditory area activation during auditory stimuli compared to healthy control patients. This study also showed that there were reductions in baseline connectivity between the left and the right primary auditory cortices, suggesting alterations in the organization and responsiveness of the cerebral cortex.[20]
Schizophrenia:
Individuals with schizophrenia display positive symptoms, including auditory hallucinations. As part of the physiological mechanism of auditory processing when an individual speaks, there is a normal speaking-induced suppression that occurs to reduce activity in the auditory cortex in response to self-generated speech so that the individual can focus more attention on sounds externally produced. Individuals with schizophrenia do not demonstrate speaking-induced suppression of the primary auditory cortex. These findings may provide insight into a mechanism behind auditory hallucinations often experienced by patients with schizophrenia.[21]