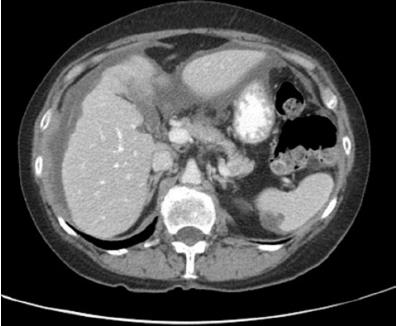
[1]
Moran BJ, Cecil TD. The etiology, clinical presentation, and management of pseudomyxoma peritonei. Surgical oncology clinics of North America. 2003 Jul:12(3):585-603
[PubMed PMID: 14567019]
[2]
Darr U, Renno A, Alkully T, Khan Z, Tiwari A, Zeb W, Purdy J, Nawras A. Diagnosis of Pseudomyxoma peritonei via endoscopic ultrasound guided fine needle aspiration: a case report and review of literature. Scandinavian journal of gastroenterology. 2017 May:52(5):609-612. doi: 10.1080/00365521.2017.1284896. Epub 2017 Feb 3
[PubMed PMID: 28155576]
Level 3 (low-level) evidence
[3]
Mittal R, Chandramohan A, Moran B. Pseudomyxoma peritonei: natural history and treatment. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2017 Aug:33(5):511-519. doi: 10.1080/02656736.2017.1310938. Epub
[PubMed PMID: 28540829]
[4]
Baratti D, Kusamura S, Milione M, Pietrantonio F, Caporale M, Guaglio M, Deraco M. Pseudomyxoma Peritonei of Extra-Appendiceal Origin: A Comparative Study. Annals of surgical oncology. 2016 Dec:23(13):4222-4230
[PubMed PMID: 27352203]
Level 2 (mid-level) evidence
[5]
Shih IM, Yan H, Speyrer D, Shmookler BM, Sugarbaker PH, Ronnett BM. Molecular genetic analysis of appendiceal mucinous adenomas in identical twins, including one with pseudomyxoma peritonei. The American journal of surgical pathology. 2001 Aug:25(8):1095-9
[PubMed PMID: 11474297]
[6]
Smeenk RM, van Velthuysen ML, Verwaal VJ, Zoetmulder FA. Appendiceal neoplasms and pseudomyxoma peritonei: a population based study. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2008 Feb:34(2):196-201
[PubMed PMID: 17524597]
[7]
García KM, Flores KM, Ruiz A, González FL, Rodríguez ÁM. Pseudomyxoma Peritonei: Case Report and Literature Review. Journal of gastrointestinal cancer. 2019 Dec:50(4):1037-1042. doi: 10.1007/s12029-018-00192-8. Epub
[PubMed PMID: 30618002]
Level 3 (low-level) evidence
[8]
Sugarbaker PH. Pseudomyxoma peritonei. A cancer whose biology is characterized by a redistribution phenomenon. Annals of surgery. 1994 Feb:219(2):109-11
[PubMed PMID: 8129480]
[9]
Sulkin TV, O'Neill H, Amin AI, Moran B. CT in pseudomyxoma peritonei: a review of 17 cases. Clinical radiology. 2002 Jul:57(7):608-13
[PubMed PMID: 12096860]
Level 3 (low-level) evidence
[10]
Pestieau SR, Esquivel J, Sugarbaker PH. Pleural extension of mucinous tumor in patients with pseudomyxoma peritonei syndrome. Annals of surgical oncology. 2000 Apr:7(3):199-203
[PubMed PMID: 10791850]
[11]
Smeenk RM, Bruin SC, van Velthuysen ML, Verwaal VJ. Pseudomyxoma peritonei. Current problems in surgery. 2008 Aug:45(8):527-75. doi: 10.1067/j.cpsurg.2008.04.003. Epub
[PubMed PMID: 18590843]
[12]
Rizvi SA, Syed W, Shergill R. Approach to pseudomyxoma peritonei. World journal of gastrointestinal surgery. 2018 Aug 27:10(5):49-56. doi: 10.4240/wjgs.v10.i5.49. Epub
[PubMed PMID: 30190782]
[13]
Sugarbaker PH, Ronnett BM, Archer A, Averbach AM, Bland R, Chang D, Dalton RR, Ettinghausen SE, Jacquet P, Jelinek J, Koslowe P, Kurman RJ, Shmookler B, Stephens AD, Steves MA, Stuart OA, White S, Zahn CM, Zoetmulder FA. Pseudomyxoma peritonei syndrome. Advances in surgery. 1996:30():233-80
[PubMed PMID: 8960339]
Level 3 (low-level) evidence
[14]
Ronnett BM, Zahn CM, Kurman RJ, Kass ME, Sugarbaker PH, Shmookler BM. Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to "pseudomyxoma peritonei". The American journal of surgical pathology. 1995 Dec:19(12):1390-408
[PubMed PMID: 7503361]
Level 3 (low-level) evidence
[15]
Ramaswamy V. Pathology of Mucinous Appendiceal Tumors and Pseudomyxoma Peritonei. Indian journal of surgical oncology. 2016 Jun:7(2):258-67. doi: 10.1007/s13193-016-0516-2. Epub 2016 Mar 19
[PubMed PMID: 27065718]
[16]
Carr NJ, Cecil TD, Mohamed F, Sobin LH, Sugarbaker PH, González-Moreno S, Taflampas P, Chapman S, Moran BJ, Peritoneal Surface Oncology Group International. A Consensus for Classification and Pathologic Reporting of Pseudomyxoma Peritonei and Associated Appendiceal Neoplasia: The Results of the Peritoneal Surface Oncology Group International (PSOGI) Modified Delphi Process. The American journal of surgical pathology. 2016 Jan:40(1):14-26. doi: 10.1097/PAS.0000000000000535. Epub
[PubMed PMID: 26492181]
Level 3 (low-level) evidence
[17]
Shetty S, Natarajan B, Thomas P, Govindarajan V, Sharma P, Loggie B. Proposed classification of pseudomyxoma peritonei: influence of signet ring cells on survival. The American surgeon. 2013 Nov:79(11):1171-6
[PubMed PMID: 24165252]
[18]
Carr NJ, Bibeau F, Bradley RF, Dartigues P, Feakins RM, Geisinger KR, Gui X, Isaac S, Milione M, Misdraji J, Pai RK, Rodriguez-Justo M, Sobin LH, van Velthuysen MF, Yantiss RK. The histopathological classification, diagnosis and differential diagnosis of mucinous appendiceal neoplasms, appendiceal adenocarcinomas and pseudomyxoma peritonei. Histopathology. 2017 Dec:71(6):847-858. doi: 10.1111/his.13324. Epub 2017 Sep 19
[PubMed PMID: 28746986]
[19]
Ferreira CR, Carvalho JP, Soares FA, Siqueira SA, Carvalho FM. Mucinous ovarian tumors associated with pseudomyxoma peritonei of adenomucinosis type: immunohistochemical evidence that they are secondary tumors. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2008 Jan-Feb:18(1):59-65
[PubMed PMID: 17511804]
Level 2 (mid-level) evidence
[20]
Fernandes ACO, Rocha GRMD, Oliveira AD, Guimarães MD, Carvalho SC, Chojniak R. Pseudomyxoma peritonei in a pediatric patient: A case report and literature review. Revista da Associacao Medica Brasileira (1992). 2018 Feb:64(2):195-199. doi: 10.1590/1806-9282.64.02.195. Epub
[PubMed PMID: 29641675]
Level 3 (low-level) evidence
[21]
Sullivan BJ, Bolton N, Sarpel U, Magge D. A unique presentation of superinfected pseudomyxoma peritonei secondary to a low-grade appendiceal mucinous neoplasm. World journal of surgical oncology. 2019 Feb 18:17(1):34. doi: 10.1186/s12957-019-1578-8. Epub 2019 Feb 18
[PubMed PMID: 30777068]
[22]
Bevan KE, Mohamed F, Moran BJ. Pseudomyxoma peritonei. World journal of gastrointestinal oncology. 2010 Jan 15:2(1):44-50. doi: 10.4251/wjgo.v2.i1.44. Epub
[PubMed PMID: 21160816]
[24]
Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, Baratti D, Deraco M, Elias D, Sardi A, Liauw W, Yan TD, Barrios P, Gómez Portilla A, de Hingh IH, Ceelen WP, Pelz JO, Piso P, González-Moreno S, Van Der Speeten K, Morris DL. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012 Jul 10:30(20):2449-56. doi: 10.1200/JCO.2011.39.7166. Epub 2012 May 21
[PubMed PMID: 22614976]
[25]
Smeenk RM, Verwaal VJ, Antonini N, Zoetmulder FA. Progression of pseudomyxoma peritonei after combined modality treatment: management and outcome. Annals of surgical oncology. 2007 Feb:14(2):493-9
[PubMed PMID: 17103067]
[26]
Stewart JH 4th, Shen P, Levine EA. Intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: current status and future directions. Annals of surgical oncology. 2005 Oct:12(10):765-77
[PubMed PMID: 16132375]
Level 3 (low-level) evidence
[27]
Blackham AU, Swett K, Eng C, Sirintrapun J, Bergman S, Geisinger KR, Votanopoulos K, Stewart JH, Shen P, Levine EA. Perioperative systemic chemotherapy for appendiceal mucinous carcinoma peritonei treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Journal of surgical oncology. 2014 Jun:109(7):740-5. doi: 10.1002/jso.23547. Epub 2013 Dec 28
[PubMed PMID: 24375188]
[28]
Baratti D, Kusamura S, Milione M, Bruno F, Guaglio M, Deraco M. Validation of the Recent PSOGI Pathological Classification of Pseudomyxoma Peritonei in a Single-Center Series of 265 Patients Treated by Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Annals of surgical oncology. 2018 Feb:25(2):404-413. doi: 10.1245/s10434-017-6252-1. Epub 2017 Nov 20
[PubMed PMID: 29159742]
Level 1 (high-level) evidence
[29]
Pallas N, Karamveri C, Kyziridis D, Hristakis C, Kyriakopoulos V, Kalakonas A, Vaikos D, Tentes AK. Cytoreductive surgery and hyperthermic intraperitenoal chemotherapy (HIPEC) for colorectal and appendiceal carcinomas with peritoneal carcinomatosis. Journal of B.U.ON. : official journal of the Balkan Union of Oncology. 2017 Nov-Dec:22(6):1547-1553
[PubMed PMID: 29332351]
[30]
McQuellon RP, Russell GB, Shen P, Stewart JH 4th, Saunders W, Levine EA. Survival and health outcomes after cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for disseminated peritoneal cancer of appendiceal origin. Annals of surgical oncology. 2008 Jan:15(1):125-33
[PubMed PMID: 18030535]
[31]
Moran B, Cecil T, Chandrakumaran K, Arnold S, Mohamed F, Venkatasubramaniam A. The results of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in 1200 patients with peritoneal malignancy. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. 2015 Sep:17(9):772-8. doi: 10.1111/codi.12975. Epub
[PubMed PMID: 25880479]
[32]
Chua TC, Yan TD, Saxena A, Morris DL. Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure?: a systematic review of morbidity and mortality. Annals of surgery. 2009 Jun:249(6):900-7. doi: 10.1097/SLA.0b013e3181a45d86. Epub
[PubMed PMID: 19474692]
Level 1 (high-level) evidence
[33]
Järvinen P, Ristimäki A, Kantonen J, Aronen M, Huuhtanen R, Järvinen H, Lepistö A. Comparison of serial debulking and cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in pseudomyxoma peritonei of appendiceal origin. International journal of colorectal disease. 2014 Aug:29(8):999-1007. doi: 10.1007/s00384-014-1933-8. Epub 2014 Jun 26
[PubMed PMID: 24965858]
[34]
Sugarbaker PH, Ryan DP. Cytoreductive surgery plus hyperthermic perioperative chemotherapy to treat peritoneal metastases from colorectal cancer: standard of care or an experimental approach? The Lancet. Oncology. 2012 Aug:13(8):e362-9. doi: 10.1016/S1470-2045(12)70210-3. Epub
[PubMed PMID: 22846841]