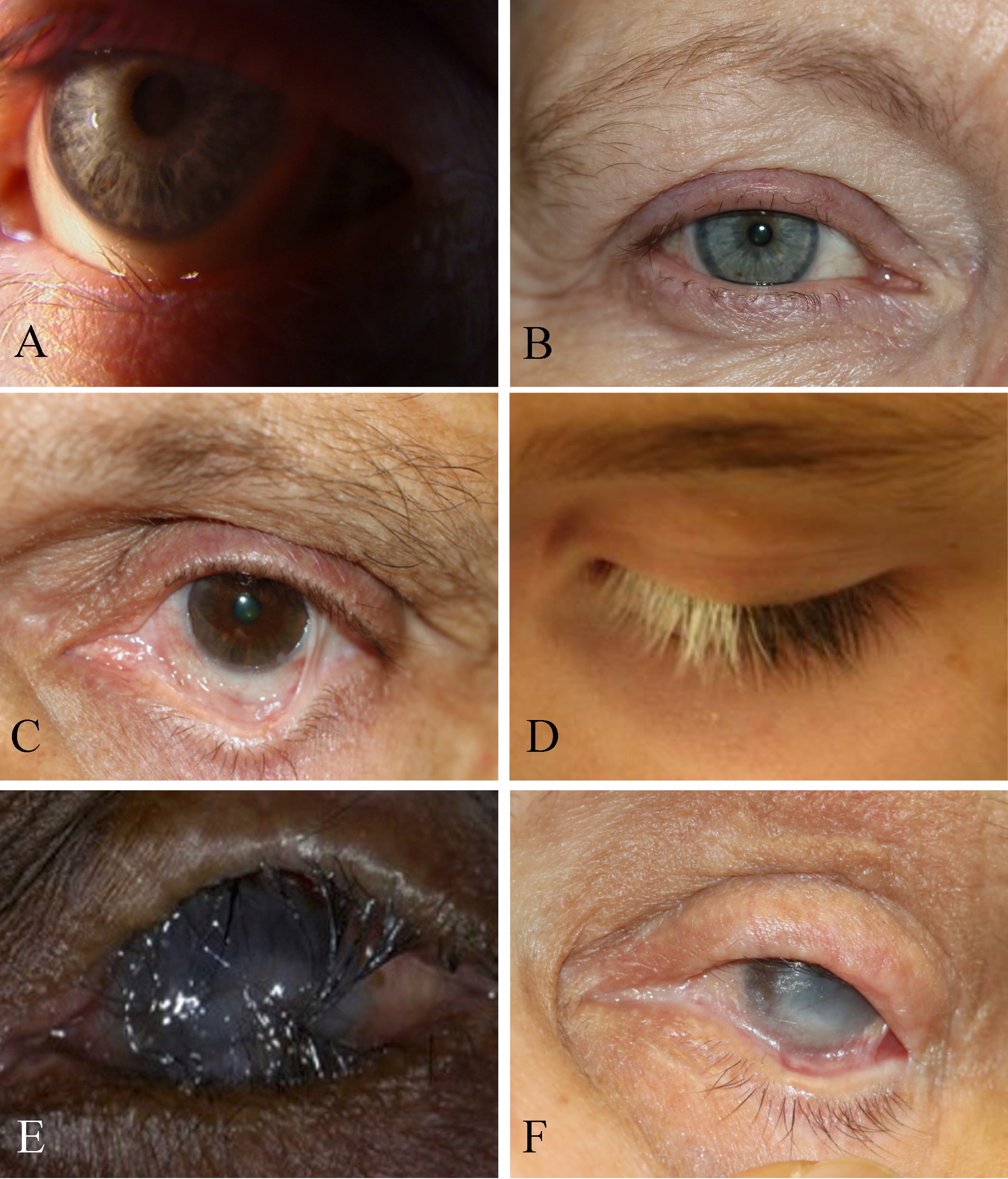
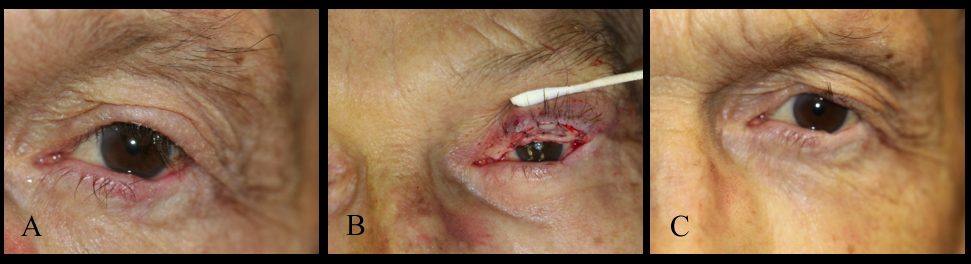
[1]
Pintea-Trifu ML, Bâlici Ş, Siserman CV, Vică ML, Matei HV. Chlamydia trachomatis and the HLA involvement in the development of infection and disease: a narrative review. Medicine and pharmacy reports. 2023 Oct:96(4):335-345. doi: 10.15386/mpr-2593. Epub 2023 Oct 26
[PubMed PMID: 37970191]
Level 3 (low-level) evidence
[2]
Harding-Esch EM, Holland MJ, Schémann JF, Sissoko M, Sarr B, Butcher RMR, Molina-Gonzalez S, Andreasen AA, Mabey DCW, Bailey RL. Facial cleanliness indicators by time of day: results of a cross-sectional trachoma prevalence survey in Senegal. Parasites & vectors. 2020 Nov 18:13(1):556. doi: 10.1186/s13071-020-04410-w. Epub 2020 Nov 18
[PubMed PMID: 33203456]
Level 2 (mid-level) evidence
[3]
Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, Das A, Jonas JB, Keeffe J, Kempen JH, Leasher J, Limburg H, Naidoo K, Pesudovs K, Silvester A, Stevens GA, Tahhan N, Wong TY, Taylor HR, Vision Loss Expert Group of the Global Burden of Disease Study. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. The Lancet. Global health. 2017 Dec:5(12):e1221-e1234. doi: 10.1016/S2214-109X(17)30393-5. Epub 2017 Oct 11
[PubMed PMID: 29032195]
Level 1 (high-level) evidence
[4]
Wang EY, Kong X, Wolle M, Gasquet N, Ssekasanvu J, Mariotti SP, Bourne R, Taylor H, Resnikoff S, West S. Global Trends in Blindness and Vision Impairment Resulting from Corneal Opacity 1984-2020: A Meta-analysis. Ophthalmology. 2023 Aug:130(8):863-871. doi: 10.1016/j.ophtha.2023.03.012. Epub 2023 Mar 22
[PubMed PMID: 36963570]
Level 1 (high-level) evidence
[5]
Quesada-Cubo V, Damián-González DC, Prado-Velasco FG, Fernández-Santos NA, Sánchez-Tejeda G, Correa-Morales F, Domínguez-Zárate H, García-Orozco A, Saboyá-Díaz MI, Sánchez-Martín MJ. The elimination of trachoma as a public health problem in Mexico: From national health priority to national success story. PLoS neglected tropical diseases. 2022 Aug:16(8):e0010660. doi: 10.1371/journal.pntd.0010660. Epub 2022 Aug 29
[PubMed PMID: 36037211]
[6]
Hadfield J, Harris SR, Seth-Smith HMB, Parmar S, Andersson P, Giffard PM, Schachter J, Moncada J, Ellison L, Vaulet MLG, Fermepin MR, Radebe F, Mendoza S, Ouburg S, Morré SA, Sachse K, Puolakkainen M, Korhonen SJ, Sonnex C, Wiggins R, Jalal H, Brunelli T, Casprini P, Pitt R, Ison C, Savicheva A, Shipitsyna E, Hadad R, Kari L, Burton MJ, Mabey D, Solomon AW, Lewis D, Marsh P, Unemo M, Clarke IN, Parkhill J, Thomson NR. Comprehensive global genome dynamics of Chlamydia trachomatis show ancient diversification followed by contemporary mixing and recent lineage expansion. Genome research. 2017 Jul:27(7):1220-1229. doi: 10.1101/gr.212647.116. Epub 2017 Jun 6
[PubMed PMID: 28588068]
[7]
Kitu M, Mihretie K, Abuhay T. Case-control study of determinants of corrective upper eyelid surgery refusals among trachomatous trichiasis patients in Ethiopia. Eastern Mediterranean health journal = La revue de sante de la Mediterranee orientale = al-Majallah al-sihhiyah li-sharq al-mutawassit. 2023 Nov 30:26(11):903-911. doi: 10.26719/emhj.23.085. Epub 2023 Nov 30
[PubMed PMID: 38279886]
Level 2 (mid-level) evidence
[8]
Kreis AJ, Nouhoum G. Comment on: Recurrent upper eyelid trachomatous entropion repair: long-term efficacy of a five-step approach. Eye (London, England). 2022 Dec:36(12):2364. doi: 10.1038/s41433-022-01974-y. Epub 2022 May 7
[PubMed PMID: 35525859]
Level 3 (low-level) evidence
[9]
Cochrane GM, Mangot M, Houinei W, Susapu M, Cama A, Le Mesurier R, Webster S, Hillgrove T, Barton J, Butcher R, Harding-Esch EM, Mabey D, Bakhtiari A, Müller A, Yajima A, Solomon AW, Kaldor J, Koim SP, Ko R, Garap J. Corneal pannus, Herbert's pits and conjunctival inflammation in older children in Papua New Guinea. Ophthalmic epidemiology. 2024 Feb 8:():1-8. doi: 10.1080/09286586.2023.2273507. Epub 2024 Feb 8
[PubMed PMID: 38329811]
[10]
Solomon AW, Burton MJ, Gower EW, Harding-Esch EM, Oldenburg CE, Taylor HR, Traoré L. Trachoma. Nature reviews. Disease primers. 2022 May 26:8(1):32. doi: 10.1038/s41572-022-00359-5. Epub 2022 May 26
[PubMed PMID: 35618795]
[11]
Genet A, Dagnew Z, Melkie G, Keleb A, Motbainor A, Mebrat A, Leshargie CT. Prevalence of active trachoma and its associated factors among 1-9 years of age children from model and non-model kebeles in Dangila district, northwest Ethiopia. PloS one. 2022:17(6):e0268441. doi: 10.1371/journal.pone.0268441. Epub 2022 Jun 15
[PubMed PMID: 35704657]
[12]
Robinson A, Gomes LRO, Abdurahman OS, Alemayehu W, Shuka G, Melese E, Guye M, Legesse D, Elias E, Temam K, Koro KH, Adugna D, Seife F, Aga MA, Sarah V, Lambert SM, Walker SL, Habtamu E, Solomon AW, Last A, Macleod D, Burton MJ, Logan JG. Evaluation of the efficacy of insecticide-treated scarves to protect children from the trachoma vector Musca sorbens (Diptera: Muscidae): A phase II randomised controlled trial in Oromia, Ethiopia. EClinicalMedicine. 2022 Jul:49():101487. doi: 10.1016/j.eclinm.2022.101487. Epub 2022 Jun 8
[PubMed PMID: 35747196]
Level 1 (high-level) evidence
[13]
Last A, Versteeg B, Shafi Abdurahman O, Robinson A, Dumessa G, Abraham Aga M, Shumi Bejiga G, Negussu N, Greenland K, Czerniewska A, Thomson N, Cairncross S, Sarah V, Macleod D, Solomon AW, Logan J, Burton MJ. Detecting extra-ocular Chlamydia trachomatis in a trachoma-endemic community in Ethiopia: Identifying potential routes of transmission. PLoS neglected tropical diseases. 2020 Mar:14(3):e0008120. doi: 10.1371/journal.pntd.0008120. Epub 2020 Mar 4
[PubMed PMID: 32130213]
[14]
Jones BR. The prevention of blindness from trachoma. Transactions of the ophthalmological societies of the United Kingdom. 1975 Apr:95(1):16-33
[PubMed PMID: 775692]
[15]
Marques AP, Ramke J, Cairns J, Butt T, Zhang JH, Jones I, Jovic M, Nandakumar A, Faal H, Taylor H, Bastawrous A, Braithwaite T, Resnikoff S, Khaw PT, Bourne R, Gordon I, Frick K, Burton MJ. The economics of vision impairment and its leading causes: A systematic review. EClinicalMedicine. 2022 Apr:46():101354. doi: 10.1016/j.eclinm.2022.101354. Epub 2022 Mar 22
[PubMed PMID: 35340626]
Level 1 (high-level) evidence
[16]
Derrick T, Roberts Ch, Last AR, Burr SE, Holland MJ. Trachoma and Ocular Chlamydial Infection in the Era of Genomics. Mediators of inflammation. 2015:2015():791847. doi: 10.1155/2015/791847. Epub 2015 Sep 3
[PubMed PMID: 26424969]
[17]
Gallenga CE, Maritati M, Del Boccio M, D'Aloisio R, Conti P, Mura M, Contini C, Gallenga PE. Why the SAFE-S Strategy for Trachoma? Are Musca sorbens or Scatophaga stercoraria Really the Culprit?-A Brief Historical Review from an Italian Point of View. Pathogens (Basel, Switzerland). 2023 Dec 4:12(12):. doi: 10.3390/pathogens12121419. Epub 2023 Dec 4
[PubMed PMID: 38133302]
[18]
Dzakah EE, Huang L, Xue Y, Wei S, Wang X, Chen H, Shui J, Kyei F, Rashid F, Zheng H, Yang B, Tang S. Host cell response and distinct gene expression profiles at different stages of Chlamydia trachomatis infection reveals stage-specific biomarkers of infection. BMC microbiology. 2021 Jan 4:21(1):3. doi: 10.1186/s12866-020-02061-6. Epub 2021 Jan 4
[PubMed PMID: 33397284]
[19]
Lynch KD, Morotti W, Brian G, Ketchup L, Kingston K, Starr M, Ware RS, Everill B, Asgar N, O'Keefe A, Whop LJ, Kaldor JM, Lambert SB. Clinical signs of trachoma and laboratory evidence of ocular Chlamydia trachomatis infection in a remote Queensland community: a serial cross-sectional study. The Medical journal of Australia. 2022 Nov 21:217(10):538-543. doi: 10.5694/mja2.51735. Epub 2022 Sep 30
[PubMed PMID: 36180097]
Level 2 (mid-level) evidence
[20]
Yang C, Kari L, Lei L, Carlson JH, Ma L, Couch CE, Whitmire WM, Bock K, Moore I, Bonner C, McClarty G, Caldwell HD. Chlamydia trachomatis Plasmid Gene Protein 3 Is Essential for the Establishment of Persistent Infection and Associated Immunopathology. mBio. 2020 Aug 18:11(4):. doi: 10.1128/mBio.01902-20. Epub 2020 Aug 18
[PubMed PMID: 32817110]
[21]
Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, Mariotti SP. Global data on visual impairment in the year 2002. Bulletin of the World Health Organization. 2004 Nov:82(11):844-51
[PubMed PMID: 15640920]
[22]
Thylefors B, Négrel AD, Pararajasegaram R, Dadzie KY. Global data on blindness. Bulletin of the World Health Organization. 1995:73(1):115-21
[PubMed PMID: 7704921]
[23]
Polack S, Brooker S, Kuper H, Mariotti S, Mabey D, Foster A. Mapping the global distribution of trachoma. Bulletin of the World Health Organization. 2005 Dec:83(12):913-9
[PubMed PMID: 16462983]
[24]
. Trachoma. Nature reviews. Disease primers. 2022 May 26:8(1):33. doi: 10.1038/s41572-022-00369-3. Epub 2022 May 26
[PubMed PMID: 35618865]
[25]
Al-Khatib T, Bella AL, Saboyá-Díaz MI, Solomon AW. Trachoma: The Last Decade? Ophthalmic epidemiology. 2023 Dec:30(6):541-543. doi: 10.1080/09286586.2023.2270045. Epub 2023 Dec 12
[PubMed PMID: 38085790]
[26]
Ono K, Umeya R. Longitudinal Analysis of Eye Health Disparities Due to Trachoma Using Country-Level Data from the Global Burden of Disease Study 2019. Ophthalmic epidemiology. 2023 Mar 7:():1-7. doi: 10.1080/09286586.2023.2188561. Epub 2023 Mar 7
[PubMed PMID: 36882966]
[27]
Impouma B, Kalu AA, Makubalo L, Gasasira A, Cabore J, Moeti M. Responding to Africa's burden of disease: accelerating progress. Epidemiology and infection. 2023 Jun 20:151():e114. doi: 10.1017/S0950268823000997. Epub 2023 Jun 20
[PubMed PMID: 37337304]
[28]
Ageed A, Khan M. Eliminating Trachoma in Africa: The Importance of Environmental Interventions. Cureus. 2024 Jan:16(1):e52358. doi: 10.7759/cureus.52358. Epub 2024 Jan 16
[PubMed PMID: 38234389]
[29]
Altaseb T, Lingerew M, Adane M. Prevalence of trachomatous inflammation-follicular and associated factors among children aged 1-9 years in northeastern Ethiopia. BMC pediatrics. 2024 Feb 19:24(1):128. doi: 10.1186/s12887-024-04587-4. Epub 2024 Feb 19
[PubMed PMID: 38373921]
[30]
Cromwell EA, Courtright P, King JD, Rotondo LA, Ngondi J, Emerson PM. The excess burden of trachomatous trichiasis in women: a systematic review and meta-analysis. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2009 Oct:103(10):985-92. doi: 10.1016/j.trstmh.2009.03.012. Epub 2009 Apr 10
[PubMed PMID: 19362326]
Level 1 (high-level) evidence
[31]
Harding-Esch EM, Burgert-Brucker CR, Jimenez C, Bakhtiari A, Willis R, Bejiga MD, Mpyet C, Ngondi J, Boyd S, Abdala M, Abdou A, Adamu Y, Alemayehu A, Alemayehu W, Al-Khatib T, Apadinuwe SC, Awaca N, Awoussi MS, Baayendag G, Badiane MD, Bailey RL, Batcho W, Bay Z, Bella A, Beido N, Bol YY, Bougouma C, Brady CJ, Bucumi V, Butcher R, Cakacaka R, Cama A, Camara M, Cassama E, Chaora SG, Chebbi AC, Chisambi AB, Chu B, Conteh A, Coulibaly SM, Courtright P, Dalmar A, Dat TM, Davids T, Djaker MEA, de Fátima Costa Lopes M, Dézoumbé D, Dodson S, Downs P, Eckman S, Elshafie BE, Elmezoghi M, Elvis AA, Emerson P, Epée EE, Faktaufon D, Fall M, Fassinou A, Fleming F, Flueckiger R, Gamael KK, Garae M, Garap J, Gass K, Gebru G, Gichangi MM, Giorgi E, Goépogui A, Gómez DVF, Gómez Forero DP, Gower EW, Harte A, Henry R, Honorio-Morales HA, Ilako DR, Issifou AAB, Jones E, Kabona G, Kabore M, Kadri B, Kalua K, Kanyi SK, Kebede S, Kebede F, Keenan JD, Kello AB, Khan AA, Khelifi H, Kilangalanga J, Kim SH, Ko R, Lewallen S, Lietman T, Logora MSY, Lopez YA, MacArthur C, Macleod C, Makangila F, Mariko B, Martin DL, Masika M, Massae P, Massangaie M, Matendechero HS, Mathewos T, McCullagh S, Meite A, Mendes EP, Abdi HM, Miller H, Minnih A, Mishra SK, Molefi T, Mosher A, M'Po N, Mugume F, Mukwiza R, Mwale C, Mwatha S, Mwingira U, Nash SD, Nassa C, Negussu N, Nieba C, Noah Noah JC, Nwosu CO, Olobio N, Opon R, Pavluck A, Phiri I, Rainima-Qaniuci M, Renneker KK, Saboyá-Díaz MI, Sakho F, Sanha S, Sarah V, Sarr B, Szwarcwald CL, Shah Salam A, Sharma S, Seife F, Serrano Chavez GM, Sissoko M, Sitoe HM, Sokana O, Tadesse F, Taleo F, Talero SL, Tarfani Y, Tefera A, Tekeraoi R, Tesfazion A, Traina A, Traoré L, Trujillo-Trujillo J, Tukahebwa EM, Vashist P, Wanyama EB, Warusavithana SDP, Watitu TK, West S, Win Y, Woods G, Yajima A, Yaya G, Zecarias A, Zewengiel S, Zoumanigui A, Hooper PJ, Millar T, Rotondo L, Solomon AW. Tropical Data: Approach and Methodology as Applied to Trachoma Prevalence Surveys. Ophthalmic epidemiology. 2023 Dec:30(6):544-560. doi: 10.1080/09286586.2023.2249546. Epub 2023 Dec 12
[PubMed PMID: 38085791]
Level 3 (low-level) evidence
[32]
Getachew D, Woldekidan F, Ayele G, Bekele Y, Sleshi S, Tekalgn E, Worku T, Ayenew M, Bogale B, Asres A. High prevalence of active trachoma and associated factors among school-aged children in Southwest Ethiopia. PLoS neglected tropical diseases. 2023 Dec:17(12):e0011846. doi: 10.1371/journal.pntd.0011846. Epub 2023 Dec 15
[PubMed PMID: 38100523]
[33]
Derrick T, Ramadhani AM, Macleod D, Massae P, Mafuru E, Aiweda M, Mbuya K, Makupa W, Mtuy T, Bailey RL, Mabey DCW, Holland MJ, Burton MJ. Immunopathogenesis of Progressive Scarring Trachoma: Results of a 4-Year Longitudinal Study in Tanzanian Children. Infection and immunity. 2020 Mar 23:88(4):. doi: 10.1128/IAI.00629-19. Epub 2020 Mar 23
[PubMed PMID: 31964744]
[34]
Tidke SC, Tidake P. A Review of Corneal Blindness: Causes and Management. Cureus. 2022 Oct:14(10):e30097. doi: 10.7759/cureus.30097. Epub 2022 Oct 9
[PubMed PMID: 36381769]
[35]
Gupta N, Yadav S, Solomon AW, Jain S, Kashyap S, Vanathi M, Tandon R. Atypical Corneal Phenotype in Patients With Trachoma and Secondary Amyloidosis. Cornea. 2022 May 1:41(5):609-615. doi: 10.1097/ICO.0000000000002791. Epub
[PubMed PMID: 34176916]
[36]
Jury B, Fleming C, Huston WM, Luu LDW. Molecular pathogenesis of Chlamydia trachomatis. Frontiers in cellular and infection microbiology. 2023:13():1281823. doi: 10.3389/fcimb.2023.1281823. Epub 2023 Oct 18
[PubMed PMID: 37920447]
[37]
Mandel C, Yang H, Buchko GW, Abendroth J, Grieshaber N, Chiarelli T, Grieshaber S, Omsland A. Expression and structure of the Chlamydia trachomatis DksA ortholog. Pathogens and disease. 2022 May 23:80(1):. doi: 10.1093/femspd/ftac007. Epub
[PubMed PMID: 35388904]
[38]
Kechagia JZ, Ezra DG, Burton MJ, Bailly M. Fibroblasts profiling in scarring trachoma identifies IL-6 as a functional component of a fibroblast-macrophage pro-fibrotic and pro-inflammatory feedback loop. Scientific reports. 2016 Jun 20:6():28261. doi: 10.1038/srep28261. Epub 2016 Jun 20
[PubMed PMID: 27321784]
[39]
Abu el-Asrar AM, Geboes K, Tabbara KF, al-Kharashi SA, Missotten L, Desmet V. Immunopathogenesis of conjunctival scarring in trachoma. Eye (London, England). 1998:12 ( Pt 3a)():453-60
[PubMed PMID: 9775249]
[40]
Abu el-Asrar AM, Geboes K, al-Kharashi SA, Tabbara KF, Missotten L. Collagen content and types in trachomatous conjunctivitis. Eye (London, England). 1998:12 ( Pt 4)():735-9
[PubMed PMID: 9850275]
[41]
al-Rajhi AA, Hidayat A, Nasr A, al-Faran M. The histopathology and the mechanism of entropion in patients with trachoma. Ophthalmology. 1993 Sep:100(9):1293-6
[PubMed PMID: 8371914]
[42]
Ghaem-Maghami S, Bailey RL, Mabey DC, Hay PE, Mahdi OS, Joof HM, Whittle HC, Ward ME, Lewis DJ. Characterization of B-cell responses to Chlamydia trachomatis antigens in humans with trachoma. Infection and immunity. 1997 Dec:65(12):4958-64
[PubMed PMID: 9393782]
[43]
Hu VH, Massae P, Weiss HA, Cree IA, Courtright P, Mabey DC, Bailey RL, Burton MJ. In vivo confocal microscopy of trachoma in relation to normal tarsal conjunctiva. Ophthalmology. 2011 Apr:118(4):747-54. doi: 10.1016/j.ophtha.2010.08.029. Epub 2010 Nov 5
[PubMed PMID: 21055819]
[44]
Ramadhani AM, Derrick T, Macleod D, Massae P, Mtuy T, Jeffries D, Roberts CH, Bailey RL, Mabey DCW, Holland MJ, Burton MJ. Immunofibrogenic Gene Expression Patterns in Tanzanian Children with Ocular Chlamydia trachomatis Infection, Active Trachoma and Scarring: Baseline Results of a 4-Year Longitudinal Study. Frontiers in cellular and infection microbiology. 2017:7():406. doi: 10.3389/fcimb.2017.00406. Epub 2017 Sep 15
[PubMed PMID: 28966918]
[45]
Lynch KD, Brian G, Ahwang T, Newie T, Newie V, Perrett C, Wharton G, Brown A, Tozer S, Kaldor JM, Whop LJ, Andrews RM, Lambert SB. Discord between presence of follicular conjunctivitis and Chlamydia trachomatis infection in a single Torres Strait Island community: a cross-sectional survey. Australian and New Zealand journal of public health. 2022 Apr:46(2):155-160. doi: 10.1111/1753-6405.13179. Epub 2022 Jan 3
[PubMed PMID: 34978363]
Level 2 (mid-level) evidence
[46]
Gallini JW, Sata E, Zerihun M, Melak B, Haile M, Zeru T, Gessese D, Ayele Z, Tadesse Z, Callahan EK, Nash SD, Weiss PS. Optimizing cluster survey designs for estimating trachomatous inflammation-follicular within trachoma control programs. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2022 Mar:116():101-107. doi: 10.1016/j.ijid.2021.12.355. Epub 2021 Dec 26
[PubMed PMID: 34965463]
Level 3 (low-level) evidence
[47]
Diallo AO, Bayissasse B, Sisay A, Seyum D, Weaver J, Munoz B, Merbs SL, Gower EW. Effectiveness of Trachomatous Trichiasis Case-identification Approaches in Ethiopia. Epidemiology (Cambridge, Mass.). 2023 Nov 1:34(6):909-920. doi: 10.1097/EDE.0000000000001656. Epub 2023 Sep 26
[PubMed PMID: 37757880]
Level 3 (low-level) evidence
[48]
Belsti Y, Fekadu SA, Assem AS. Active trachoma prevalence and its associated factors among children aged 1-9 years in rural residents of Lare District, Southwest Ethiopia. International journal of ophthalmology. 2021:14(11):1756-1764. doi: 10.18240/ijo.2021.11.16. Epub 2021 Nov 18
[PubMed PMID: 34804867]
[49]
Delelegn D, Tolcha A, Beyene H, Tsegaye B. Status of active trachoma infection among school children who live in villages of open field defecation: a comparative cross-sectional study. BMC public health. 2021 Nov 9:21(1):2051. doi: 10.1186/s12889-021-12106-8. Epub 2021 Nov 9
[PubMed PMID: 34753484]
Level 2 (mid-level) evidence
[50]
Mustafa O, Daoud YJ. Herbert Pits in Trachoma Infection. Mayo Clinic proceedings. 2020 Jan:95(1):134-135. doi: 10.1016/j.mayocp.2019.10.027. Epub
[PubMed PMID: 31902408]
[51]
Wang Y, Yuan Y, Pang L, Qiu B, Su D, Guan X, Xiang X, Li J. The upper eyelid levator weakening procedure for the correction of severe cicatricial entropion caused by trachoma. Annals of palliative medicine. 2020 Nov:9(6):4113-4118. doi: 10.21037/apm-20-2067. Epub
[PubMed PMID: 33302671]
Level 2 (mid-level) evidence
[52]
Schachter J, Moncada J, Dawson CR, Sheppard J, Courtright P, Said ME, Zaki S, Hafez SF, Lorincz A. Nonculture methods for diagnosing chlamydial infection in patients with trachoma: a clue to the pathogenesis of the disease? The Journal of infectious diseases. 1988 Dec:158(6):1347-52
[PubMed PMID: 3058819]
[53]
Solomon AW, Peeling RW, Foster A, Mabey DC. Diagnosis and assessment of trachoma. Clinical microbiology reviews. 2004 Oct:17(4):982-1011, table of contents
[PubMed PMID: 15489358]
[54]
Solomon AW, Kello AB, Bangert M, West SK, Taylor HR, Tekeraoi R, Foster A. The simplified trachoma grading system, amended. Bulletin of the World Health Organization. 2020 Oct 1:98(10):698-705. doi: 10.2471/BLT.19.248708. Epub 2020 Sep 3
[PubMed PMID: 33177759]
[55]
Naufal F, West SK, Brady CJ. Utility of photography for trachoma surveys: A systematic review. Survey of ophthalmology. 2022 May-Jun:67(3):842-857. doi: 10.1016/j.survophthal.2021.08.005. Epub 2021 Aug 20
[PubMed PMID: 34425127]
Level 1 (high-level) evidence
[56]
Socia D, Brady CJ, West SK, Cockrell RC. Detection of trachoma using machine learning approaches. PLoS neglected tropical diseases. 2022 Dec:16(12):e0010943. doi: 10.1371/journal.pntd.0010943. Epub 2022 Dec 7
[PubMed PMID: 36477293]
[57]
Milad D, Antaki F, Robert MC, Duval R. Development and deployment of a smartphone application for diagnosing trachoma: Leveraging code-free deep learning and edge artificial intelligence. Saudi journal of ophthalmology : official journal of the Saudi Ophthalmological Society. 2023 Jul-Sep:37(3):200-206. doi: 10.4103/sjopt.sjopt_106_22. Epub 2023 Feb 16
[PubMed PMID: 38074296]
[58]
West SK. Milestones in the fight to eliminate trachoma. Ophthalmic & physiological optics : the journal of the British College of Ophthalmic Opticians (Optometrists). 2020 Mar:40(2):66-74. doi: 10.1111/opo.12666. Epub 2020 Feb 3
[PubMed PMID: 32017172]
[59]
Sanders AM, Makoy S, Deathe AR, Ohidor S, Jesudason TC, Nute AW, Odongi P, Boniface L, Abuba S, Delahaut AS, Sebit W, Niquette J, Callahan EK, Walker DG, Nash SD. Cost and community acceptability of enhanced antibiotic distribution approaches for trachoma in the Republic of South Sudan: enhancing the A in SAFE (ETAS) study protocol. BMC ophthalmology. 2023 Feb 6:23(1):51. doi: 10.1186/s12886-023-02783-x. Epub 2023 Feb 6
[PubMed PMID: 36747194]
[60]
Muche N, Wasihun Y, Wondiye H, Bogale EK, Anagaw TF. Behavioral Responses for Face Cleanliness Message to Prevent Trachoma Among Mothers Having Children Age 1-9 Years Old, in Fogera District, Northwest Ethiopia: An Application of Extended Parallel Process Model. International journal of general medicine. 2023:16():1927-1941. doi: 10.2147/IJGM.S412380. Epub 2023 May 19
[PubMed PMID: 37228742]
[61]
Lakew S, Asefa G, Zerdo Z. Assessment of the status of improved F&E trachoma control practices among children of agro-pastoralists in Southern Ethiopia: a mixed design survey using theory of triadic influences. BMC public health. 2023 Mar 23:23(1):556. doi: 10.1186/s12889-023-15438-9. Epub 2023 Mar 23
[PubMed PMID: 36959544]
Level 3 (low-level) evidence
[62]
Gower E, Bayissasse B, Kello AB, Jesudason T. Maintaining high quality trichiasis surgery before and after trachoma elimination. Community eye health. 2023:36(120):17
[PubMed PMID: 38178827]
Level 2 (mid-level) evidence
[63]
Wang LA, Lai CC. "Etiology of trichiasis/distichiasis and its management with CO2 laser ablation". Plastic and reconstructive surgery. 2023 Oct 3:():. doi: 10.1097/PRS.0000000000011107. Epub 2023 Oct 3
[PubMed PMID: 37797242]
[64]
Habtamu E, Wondie T, Gobezie W, Tadesse Z, Gashaw B, Gebeyehu A, Roberts CH, Callahan EK, Macleod D, Burton MJ. Effect of repeated epilation for minor trachomatous trichiasis on lash burden, phenotype and surgical management willingness: A cohort study. PLoS neglected tropical diseases. 2020 Dec:14(12):e0008882. doi: 10.1371/journal.pntd.0008882. Epub 2020 Dec 14
[PubMed PMID: 33315876]
[65]
Mwangi G, Courtright P, Solomon AW. National approaches to trichiasis surgical follow-up, outcome assessment and surgeon audit in trachoma-endemic countries in Africa. The British journal of ophthalmology. 2021 Jul:105(7):904-908. doi: 10.1136/bjophthalmol-2019-315777. Epub 2020 Jul 26
[PubMed PMID: 32713838]
[66]
Kreis AJ, Gower EW, Kropp M, Kello AB, Nouhoum G, Resnikoff S, Talero SL, Solomon AW. The prevention and management of postoperative trachomatous trichiasis: A systematic review. Survey of ophthalmology. 2024 Jan-Feb:69(1):93-102. doi: 10.1016/j.survophthal.2023.02.008. Epub 2023 Mar 5
[PubMed PMID: 36878359]
Level 1 (high-level) evidence
[67]
Hailemariam B, Sata E, Halefom M, Deathe AR, Zerihun M, Jensen KA, Callahan EK, Beyene M, Adriaensen W, Owiti P, Senkoro M, Zolfo M, Nash SD. Surgical output within the Fast Track Initiative against trachoma in Amhara region, Ethiopia. Journal of infection in developing countries. 2022 Aug 31:16(8.1):8S-14S. doi: 10.3855/jidc.15978. Epub 2022 Aug 31
[PubMed PMID: 36156496]
[68]
Muthiah S, Radhakrishnan N. Management of Extraocular Infections. Indian journal of pediatrics. 2017 Dec:84(12):945-952. doi: 10.1007/s12098-017-2409-y. Epub 2017 Jul 14
[PubMed PMID: 28707045]
[69]
Thylefors B, Dawson CR, Jones BR, West SK, Taylor HR. A simple system for the assessment of trachoma and its complications. Bulletin of the World Health Organization. 1987:65(4):477-83
[PubMed PMID: 3500800]
[70]
Ngondi J, Onsarigo A, Matthews F, Reacher M, Brayne C, Baba S, Solomon AW, Zingeser J, Emerson PM. Effect of 3 years of SAFE (surgery, antibiotics, facial cleanliness, and environmental change) strategy for trachoma control in southern Sudan: a cross-sectional study. Lancet (London, England). 2006 Aug 12:368(9535):589-95
[PubMed PMID: 16905023]
Level 2 (mid-level) evidence
[71]
Asmare ZA, Assefa NL, Abebe D, Nigatu SG, Alimaw YA. Trachoma prevention practice and associated factors among mothers having children aged under nine years in Andabet district, northwest Ethiopia, 2022: A multi-level analysis. PLoS neglected tropical diseases. 2023 Jun:17(6):e0011433. doi: 10.1371/journal.pntd.0011433. Epub 2023 Jun 30
[PubMed PMID: 37390045]
[72]
Ciciriello AM, Addiss DG, Teferi T, Emerson PM, Hooper PJ, Seid M, Tadesse G, Seife F, Sormolo MJ, Kebede F, Kiflu G, West SK, Alemu M, LaCon G, Gebre T. An observational assessment of the safety of mass drug administration for trachoma in Ethiopian children. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2022 Oct 2:116(10):917-923. doi: 10.1093/trstmh/trac006. Epub
[PubMed PMID: 35106593]
[73]
. WHO Alliance for the Global Elimination of Trachoma by 2020: progress report on elimination of trachoma, 2014–2016. Releve epidemiologique hebdomadaire. 2017 Jun 30:92(26):359-68
[PubMed PMID: 28664685]
[74]
Larkin HD. WHO Program May Eliminate Active Trachoma's Blindness Risk by 2030. JAMA. 2022 May 17:327(19):1859. doi: 10.1001/jama.2022.7383. Epub
[PubMed PMID: 35579636]
[75]
Mahmud H, Landskroner E, Amza A, Aragie S, Godwin WW, de Hostos Barth A, O'Brien KS, Lietman TM, Oldenburg CE. Stopping azithromycin mass drug administration for trachoma: A systematic review. PLoS neglected tropical diseases. 2021 Jul:15(7):e0009491. doi: 10.1371/journal.pntd.0009491. Epub 2021 Jul 8
[PubMed PMID: 34237074]
Level 1 (high-level) evidence
[76]
Astale T, Ebert CD, Nute AW, Zerihun M, Gessese D, Melak B, Sata E, Ayele Z, Ayenew G, Callahan EK, Haile M, Zeru T, Tadesse Z, Nash SD. The population-based prevalence of trachomatous scarring in a trachoma hyperendemic setting: results from 152 impact surveys in Amhara, Ethiopia. BMC ophthalmology. 2021 May 13:21(1):213. doi: 10.1186/s12886-021-01972-w. Epub 2021 May 13
[PubMed PMID: 33985443]
Level 3 (low-level) evidence
[77]
Wu TJ, Reynolds MM. Trachoma, the world's leading infectious cause of blindness: The remaining gap in care and access to basic handwashing facilities. European journal of ophthalmology. 2023 Jul:33(4):1576-1582. doi: 10.1177/11206721231154295. Epub 2023 Feb 1
[PubMed PMID: 36726295]