[1]
Delle Grottaglie B, Girotti F, Ghisolfi A, Tafi A, Pescia M. A case of carcinomatous meningitis with papilledema as the only symptom: favorable response to intrathecal chemotherapy. Italian journal of neurological sciences. 1983 Apr:4(1):95-7
[PubMed PMID: 6688066]
Level 3 (low-level) evidence
[2]
Wang N, Bertalan MS, Brastianos PK. Leptomeningeal metastasis from systemic cancer: Review and update on management. Cancer. 2018 Jan 1:124(1):21-35. doi: 10.1002/cncr.30911. Epub 2017 Nov 22
[PubMed PMID: 29165794]
[3]
Le Rhun E, Taillibert S, Chamberlain MC. Carcinomatous meningitis: Leptomeningeal metastases in solid tumors. Surgical neurology international. 2013:4(Suppl 4):S265-88. doi: 10.4103/2152-7806.111304. Epub 2013 May 2
[PubMed PMID: 23717798]
[4]
Clarke JL, Perez HR, Jacks LM, Panageas KS, Deangelis LM. Leptomeningeal metastases in the MRI era. Neurology. 2010 May 4:74(18):1449-54. doi: 10.1212/WNL.0b013e3181dc1a69. Epub
[PubMed PMID: 20439847]
[5]
Thakkar JP, Kumthekar P, Dixit KS, Stupp R, Lukas RV. Leptomeningeal metastasis from solid tumors. Journal of the neurological sciences. 2020 Apr 15:411():116706. doi: 10.1016/j.jns.2020.116706. Epub 2020 Jan 23
[PubMed PMID: 32007755]
[6]
Moosavi L, D'Assumpcao C, Bowen J, Heidari A, Cobos E. Leptomeningeal Carcinomatosis From Carcinoma of Unknown Primary in a Young Patient: A Case Report and a Literature Review. Journal of investigative medicine high impact case reports. 2019 Jan-Dec:7():2324709619869380. doi: 10.1177/2324709619869380. Epub
[PubMed PMID: 31423841]
Level 3 (low-level) evidence
[7]
McGuire S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Advances in nutrition (Bethesda, Md.). 2016 Mar:7(2):418-9. doi: 10.3945/an.116.012211. Epub 2016 Mar 15
[PubMed PMID: 26980827]
Level 3 (low-level) evidence
[8]
GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet (London, England). 2016 Oct 8:388(10053):1459-1544. doi: 10.1016/S0140-6736(16)31012-1. Epub
[PubMed PMID: 27733281]
Level 1 (high-level) evidence
[9]
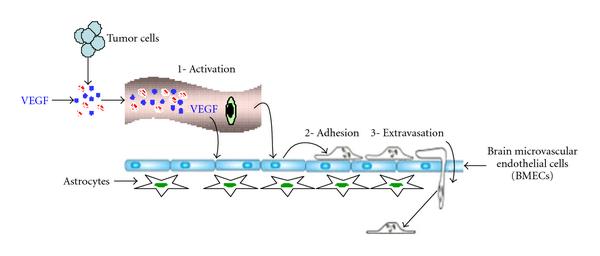
Arshad F, Wang L, Sy C, Avraham S, Avraham HK. Blood-brain barrier integrity and breast cancer metastasis to the brain. Pathology research international. 2010 Dec 29:2011():920509. doi: 10.4061/2011/920509. Epub 2010 Dec 29
[PubMed PMID: 21253507]
[10]
Sagar SM. Carcinomatous Meningitis: it does not have to be a death sentence. Oncology (Williston Park, N.Y.). 2002 Feb:16(2):237-43; discussion 244, 249-50
[PubMed PMID: 11866138]
[11]
Picarelli H, Oliveira ML, Marta GN, Solla DJF, Teixeira MJ, Figueiredo EG. Mortality, Morbidity, and Prognostic Factors in the Surgical Resection of Brain Metastases: A Contemporary Cohort Study. Journal of neurological surgery. Part A, Central European neurosurgery. 2020 Jul:81(4):279-289. doi: 10.1055/s-0039-1696997. Epub 2020 Feb 27
[PubMed PMID: 32107755]
[12]
Ma R, Levy M, Gui B, Lu SE, Narra V, Goyal S, Danish S, Hanft S, Khan AJ, Malhotra J, Motwani S, Jabbour SK. Risk of leptomeningeal carcinomatosis in patients with brain metastases treated with stereotactic radiosurgery. Journal of neuro-oncology. 2018 Jan:136(2):395-401. doi: 10.1007/s11060-017-2666-7. Epub 2017 Nov 20
[PubMed PMID: 29159778]
[13]
Jung JM, Kim S, Joo J, Shin KH, Gwak HS, Lee SH. Incidence and risk factors for leptomeningeal carcinomatosis in breast cancer patients with parenchymal brain metastases. Journal of Korean Neurosurgical Society. 2012 Sep:52(3):193-9. doi: 10.3340/jkns.2012.52.3.193. Epub 2012 Sep 30
[PubMed PMID: 23115660]
[14]
Huang AJ, Huang KE, Page BR, Ayala-Peacock DN, Lucas JT Jr, Lesser GJ, Laxton AW, Tatter SB, Chan MD. Risk factors for leptomeningeal carcinomatosis in patients with brain metastases who have previously undergone stereotactic radiosurgery. Journal of neuro-oncology. 2014 Oct:120(1):163-9. doi: 10.1007/s11060-014-1539-6. Epub 2014 Jul 22
[PubMed PMID: 25048529]
[15]
Lara-Medina F, Crismatt A, Villarreal-Garza C, Alvarado-Miranda A, Flores-Hernández L, González-Pinedo M, Gamboa-Vignolle C, Ruiz-González JD, Arrieta O. Clinical features and prognostic factors in patients with carcinomatous meningitis secondary to breast cancer. The breast journal. 2012 May-Jun:18(3):233-41. doi: 10.1111/j.1524-4741.2012.01228.x. Epub 2012 Apr 5
[PubMed PMID: 22487060]
[16]
Clarke JL. Leptomeningeal metastasis from systemic cancer. Continuum (Minneapolis, Minn.). 2012 Apr:18(2):328-42. doi: 10.1212/01.CON.0000413661.58045.e7. Epub
[PubMed PMID: 22810130]
[17]
Rigakos G, Liakou CI, Felipe N, Orkoulas-Razis D, Razis E. Clinical Presentation, Diagnosis, and Radiological Findings of Neoplastic Meningitis. Cancer control : journal of the Moffitt Cancer Center. 2017 Jan:24(1):9-21
[PubMed PMID: 28178708]
[18]
Waki F, Ando M, Takashima A, Yonemori K, Nokihara H, Miyake M, Tateishi U, Tsuta K, Shimada Y, Fujiwara Y, Tamura T. Prognostic factors and clinical outcomes in patients with leptomeningeal metastasis from solid tumors. Journal of neuro-oncology. 2009 Jun:93(2):205-12. doi: 10.1007/s11060-008-9758-3. Epub 2008 Nov 29
[PubMed PMID: 19043775]
Level 2 (mid-level) evidence
[19]
Taillibert S, Laigle-Donadey F, Chodkiewicz C, Sanson M, Hoang-Xuan K, Delattre JY. Leptomeningeal metastases from solid malignancy: a review. Journal of neuro-oncology. 2005 Oct:75(1):85-99
[PubMed PMID: 16215819]
[20]
Nayar G, Ejikeme T, Chongsathidkiet P, Elsamadicy AA, Blackwell KL, Clarke JM, Lad SP, Fecci PE. Leptomeningeal disease: current diagnostic and therapeutic strategies. Oncotarget. 2017 Sep 22:8(42):73312-73328. doi: 10.18632/oncotarget.20272. Epub 2017 Aug 16
[PubMed PMID: 29069871]
[21]
Foo CT, Burrell LM, Johnson DF. An unusual presentation of carcinomatous meningitis. Oxford medical case reports. 2016 Aug:2016(8):omw068. doi: 10.1093/omcr/omw068. Epub 2016 Aug 25
[PubMed PMID: 27574561]
Level 3 (low-level) evidence
[22]
Chamberlain MC, Corey-Bloom J. Leptomeningeal metastases: 111indium-DTPA CSF flow studies. Neurology. 1991 Nov:41(11):1765-9
[PubMed PMID: 1944906]
[23]
Taillibert S, Chamberlain MC. Leptomeningeal metastasis. Handbook of clinical neurology. 2018:149():169-204. doi: 10.1016/B978-0-12-811161-1.00013-X. Epub
[PubMed PMID: 29307353]
[24]
Bigner SH. Cerebrospinal fluid (CSF) cytology: current status and diagnostic applications. Journal of neuropathology and experimental neurology. 1992 May:51(3):235-45
[PubMed PMID: 1583530]
[25]
Malkin MG, Posner JB. Cerebrospinal fluid tumor markers for the diagnosis and management of leptomeningeal metastases. European journal of cancer & clinical oncology. 1987 Jan:23(1):1-4
[PubMed PMID: 3297711]
[26]
Corsini E, Bernardi G, Gaviani P, Silvani A, de Grazia U, Ciusani E, Croci D, Salmaggi A. Intrathecal synthesis of tumor markers is a highly sensitive test in the diagnosis of leptomeningeal metastasis from solid cancers. Clinical chemistry and laboratory medicine. 2009:47(7):874-9. doi: 10.1515/CCLM.2009.183. Epub
[PubMed PMID: 19453289]
[27]
Bruna J, González L, Miró J, Velasco R, Gil M, Tortosa A, Neuro-Oncology Unit of the Institute of Biomedical Investigation of Bellvitge. Leptomeningeal carcinomatosis: prognostic implications of clinical and cerebrospinal fluid features. Cancer. 2009 Jan 15:115(2):381-9. doi: 10.1002/cncr.24041. Epub
[PubMed PMID: 19109820]
[28]
Warley F, Bonella MB, Giunta DH, Elizondo CM, Ferreyro BL. [Associated factors with the presence of secondary neoplastic cells in the cerebrospinal fluid of patients with suspected carcinomatous meningitis]. Revista de la Facultad de Ciencias Medicas (Cordoba, Argentina). 2017:74(1):26-32
[PubMed PMID: 28379128]
[29]
Grossman SA, Trump DL, Chen DC, Thompson G, Camargo EE. Cerebrospinal fluid flow abnormalities in patients with neoplastic meningitis. An evaluation using 111indium-DTPA ventriculography. The American journal of medicine. 1982 Nov:73(5):641-7
[PubMed PMID: 6814249]
[30]
Glantz MJ, Hall WA, Cole BF, Chozick BS, Shannon CM, Wahlberg L, Akerley W, Marin L, Choy H. Diagnosis, management, and survival of patients with leptomeningeal cancer based on cerebrospinal fluid-flow status. Cancer. 1995 Jun 15:75(12):2919-31
[PubMed PMID: 7773943]
[31]
Freilich RJ, Krol G, DeAngelis LM. Neuroimaging and cerebrospinal fluid cytology in the diagnosis of leptomeningeal metastasis. Annals of neurology. 1995 Jul:38(1):51-7
[PubMed PMID: 7611725]
[32]
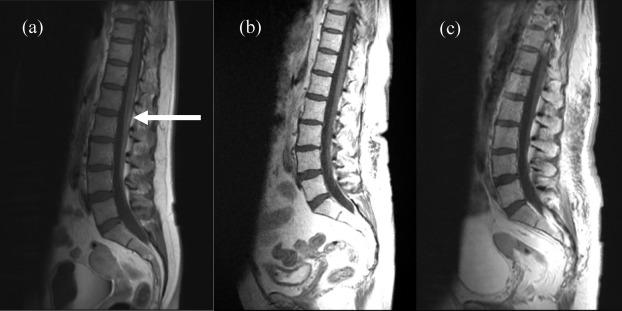
Ko Y, Gwak HS, Park EY, Joo J, Lee YJ, Lee SH, Kwon JW, Shin SH, Yoo H. Association of MRI findings with clinical characteristics and prognosis in patients with leptomeningeal carcinomatosis from non-small cell lung cancer. Journal of neuro-oncology. 2019 Jul:143(3):553-562. doi: 10.1007/s11060-019-03190-3. Epub 2019 May 14
[PubMed PMID: 31089925]
[33]
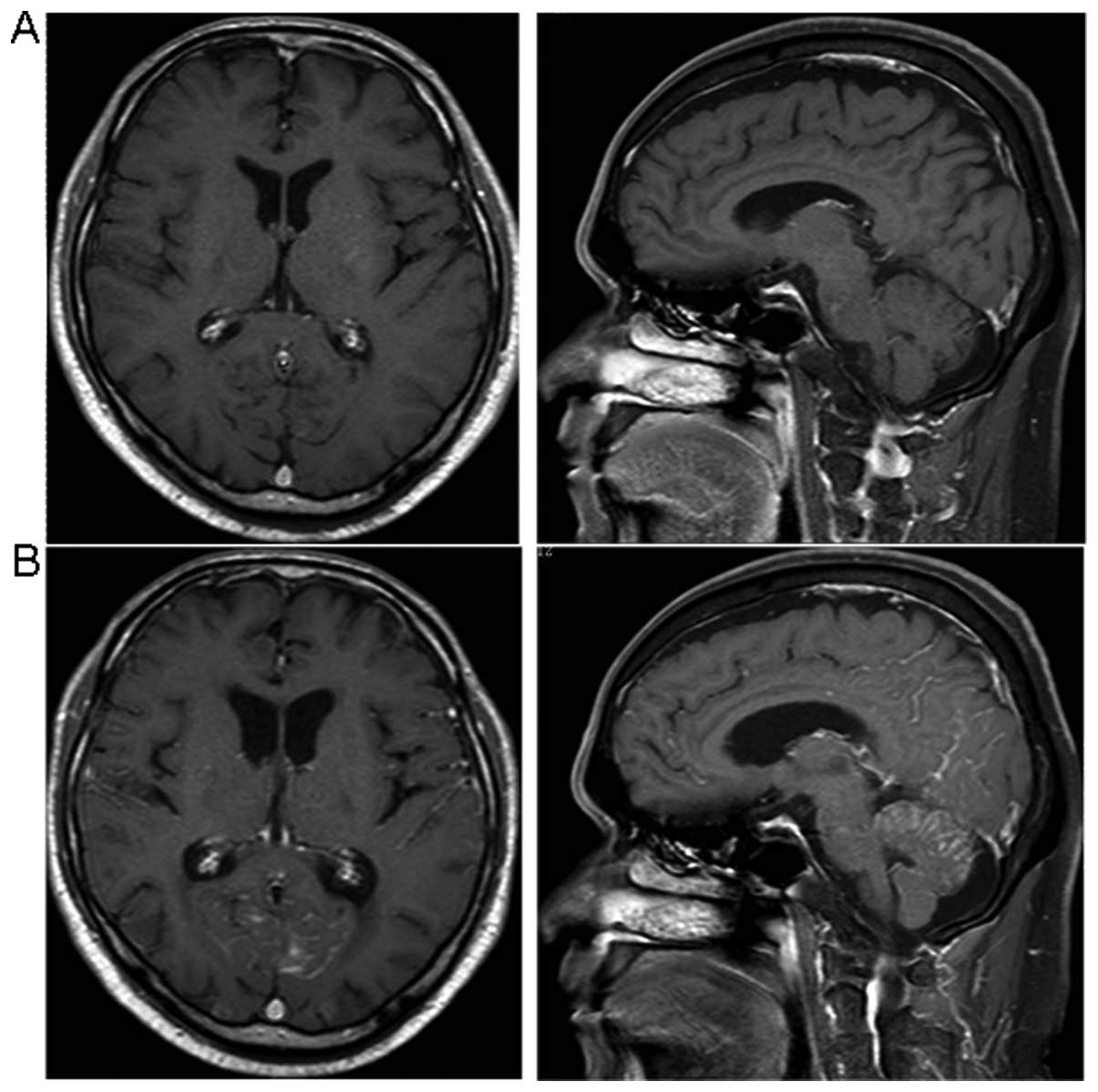
Bier G, Klumpp B, Roder C, Garbe C, Preibsch H, Ernemann U, Hempel JM. Meningeal enhancement depicted by magnetic resonance imaging in tumor patients: neoplastic meningitis or therapy-related enhancement? Neuroradiology. 2019 Jul:61(7):775-782. doi: 10.1007/s00234-019-02215-y. Epub 2019 Apr 18
[PubMed PMID: 31001647]
[34]
Kordbacheh T, Law WY, Smith IE. Sanctuary site leptomeningeal metastases in HER-2 positive breast cancer: A review in the era of trastuzumab. Breast (Edinburgh, Scotland). 2016 Apr:26():54-8. doi: 10.1016/j.breast.2015.11.005. Epub 2016 Jan 22
[PubMed PMID: 27017242]
[35]
Oguri T, Uemura T, Kunii E, Ozasa H, Ohkubo H, Miyazaki M, Maeno K, Sato S. A case of carcinomatous meningitis despite prophylactic cranial irradiation in small cell lung cancer during treatment with amrubicin. Oncology letters. 2012 Aug:4(2):305-306
[PubMed PMID: 22844374]
Level 3 (low-level) evidence
[36]
El Shafie RA, Böhm K, Weber D, Lang K, Schlaich F, Adeberg S, Paul A, Haefner MF, Katayama S, Hörner-Rieber J, Hoegen P, Löw S, Debus J, Rieken S, Bernhardt D. Palliative Radiotherapy for Leptomeningeal Carcinomatosis-Analysis of Outcome, Prognostic Factors, and Symptom Response. Frontiers in oncology. 2018:8():641. doi: 10.3389/fonc.2018.00641. Epub 2019 Jan 8
[PubMed PMID: 30671384]
[37]
Roth P, Weller M. Management of neoplastic meningitis. Chinese clinical oncology. 2015 Jun:4(2):26. doi: 10.3978/j.issn.2304-3865.2015.05.02. Epub
[PubMed PMID: 26112812]
[38]
Chang EL, Maor MH. Standard and novel radiotherapeutic approaches to neoplastic meningitis. Current oncology reports. 2003 Jan:5(1):24-8
[PubMed PMID: 12493147]
[39]
Buszek SM, Chung C. Radiotherapy in Leptomeningeal Disease: A Systematic Review of Randomized and Non-randomized Trials. Frontiers in oncology. 2019:9():1224. doi: 10.3389/fonc.2019.01224. Epub 2019 Nov 15
[PubMed PMID: 31803614]
Level 2 (mid-level) evidence
[40]
Hitchins RN, Bell DR, Woods RL, Levi JA. A prospective randomized trial of single-agent versus combination chemotherapy in meningeal carcinomatosis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1987 Oct:5(10):1655-62
[PubMed PMID: 3309199]
Level 1 (high-level) evidence
[41]
Glantz MJ, Van Horn A, Fisher R, Chamberlain MC. Route of intracerebrospinal fluid chemotherapy administration and efficacy of therapy in neoplastic meningitis. Cancer. 2010 Apr 15:116(8):1947-52. doi: 10.1002/cncr.24921. Epub
[PubMed PMID: 20151421]
[42]
Leal T, Chang JE, Mehta M, Robins HI. Leptomeningeal Metastasis: Challenges in Diagnosis and Treatment. Current cancer therapy reviews. 2011 Nov:7(4):319-327
[PubMed PMID: 23251128]
Level 3 (low-level) evidence
[43]
Maur M, Omarini C, Piacentini F, Fontana A, Pettorelli E, Cascinu S. Metronomic Capecitabine Effectively Blocks Leptomeningeal Carcinomatosis From Breast Cancer: A Case Report and Literature Review. The American journal of case reports. 2017 Feb 28:18():208-211
[PubMed PMID: 28242865]
Level 3 (low-level) evidence
[44]
Morichika D, Kubo T, Gotoda H, Tamura T, Ohashi K, Hotta K, Tabata M, Kurozumi K, Tanimoto M, Kiura K. Efficacy of multimodal treatment for leptomeningeal metastases in a lung cancer harboring an EGFR mutation. OncoTargets and therapy. 2016:9():1753-8. doi: 10.2147/OTT.S95721. Epub 2016 Mar 22
[PubMed PMID: 27042125]
[45]
Duchnowska R, Loibl S, Jassem J. Tyrosine kinase inhibitors for brain metastases in HER2-positive breast cancer. Cancer treatment reviews. 2018 Jun:67():71-77. doi: 10.1016/j.ctrv.2018.05.004. Epub 2018 May 9
[PubMed PMID: 29772459]
[46]
Kim DW, Barcena E, Mehta UN, Rohlfs ML, Kumar AJ, Penas-Prado M, Kim KB. Prolonged survival of a patient with metastatic leptomeningeal melanoma treated with BRAF inhibition-based therapy: a case report. BMC cancer. 2015 May 13:15():400. doi: 10.1186/s12885-015-1391-x. Epub 2015 May 13
[PubMed PMID: 25962795]
Level 3 (low-level) evidence
[47]
Li G, Dai WR, Shao FC. Effect of ALK-inhibitors in the treatment of non-small cell lung cancer: a systematic review and meta-analysis. European review for medical and pharmacological sciences. 2017 Aug:21(15):3496-3503
[PubMed PMID: 28829490]
Level 1 (high-level) evidence
[48]
Li N, Yang BY, Li JL, Zhu JQ, Zou BH, Wang YF, Yu L, Yao XY. [Clinical features and prognostic factors in patients with leptomeningeal metastases]. Zhonghua zhong liu za zhi [Chinese journal of oncology]. 2013 Nov:35(11):867-70
[PubMed PMID: 24447488]
[49]
Mrugala MM, Kim B, Sharma A, Johnson N, Graham C, Kurland BF, Gralow J. Phase II Study of Systemic High-dose Methotrexate and Intrathecal Liposomal Cytarabine for Treatment of Leptomeningeal Carcinomatosis From Breast Cancer. Clinical breast cancer. 2019 Oct:19(5):311-316. doi: 10.1016/j.clbc.2019.04.004. Epub 2019 Apr 18
[PubMed PMID: 31175053]
[50]
Lee SJ, Lee JI, Nam DH, Ahn YC, Han JH, Sun JM, Ahn JS, Park K, Ahn MJ. Leptomeningeal carcinomatosis in non-small-cell lung cancer patients: impact on survival and correlated prognostic factors. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2013 Feb:8(2):185-91. doi: 10.1097/JTO.0b013e3182773f21. Epub
[PubMed PMID: 23328548]
[51]
Hyun JW, Jeong IH, Joung A, Cho HJ, Kim SH, Kim HJ. Leptomeningeal metastasis: Clinical experience of 519 cases. European journal of cancer (Oxford, England : 1990). 2016 Mar:56():107-114. doi: 10.1016/j.ejca.2015.12.021. Epub 2016 Feb 1
[PubMed PMID: 26841095]
Level 3 (low-level) evidence
[52]
DeAngelis LM. Current diagnosis and treatment of leptomeningeal metastasis. Journal of neuro-oncology. 1998 Jun-Jul:38(2-3):245-52
[PubMed PMID: 9696379]