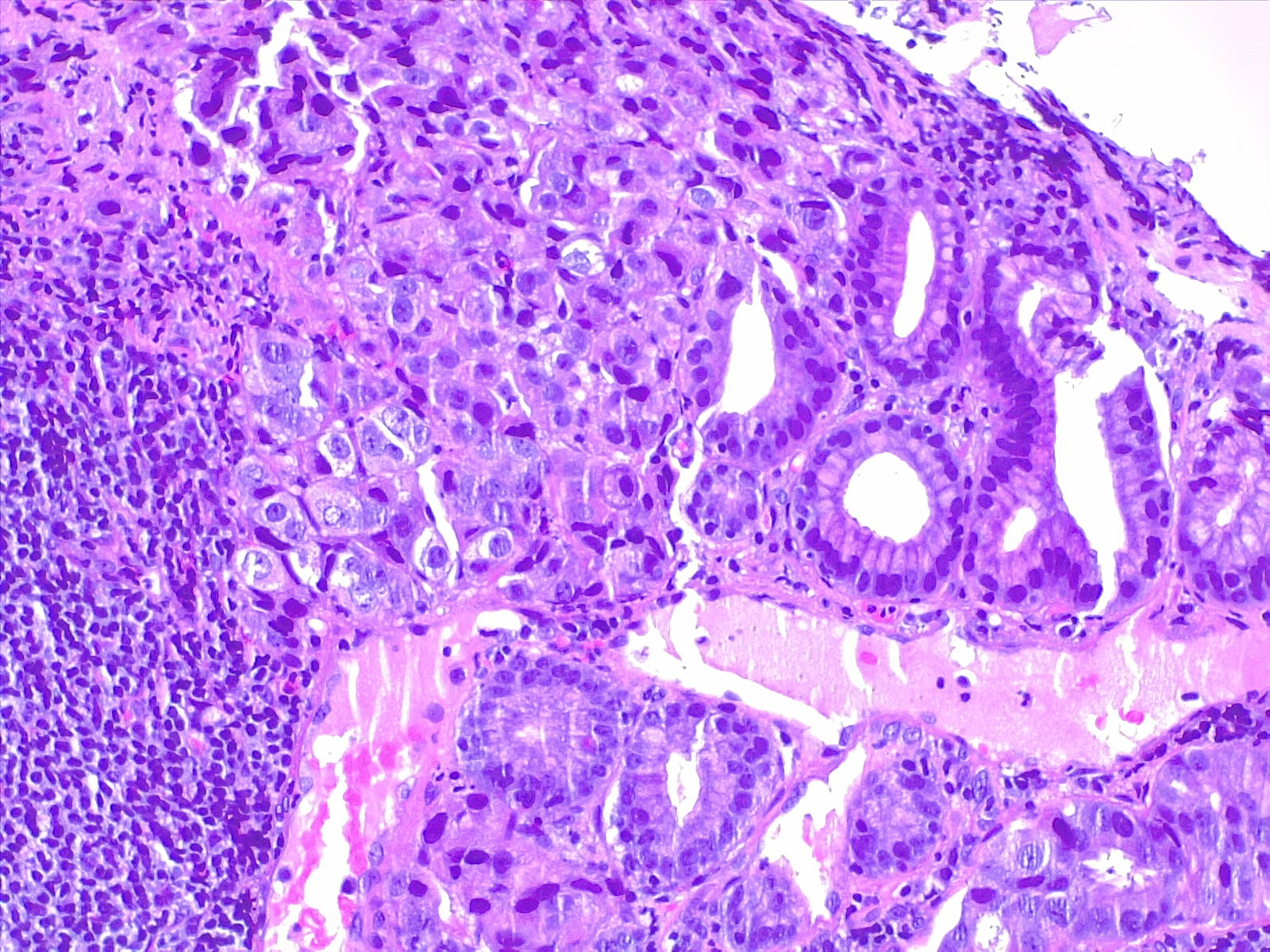
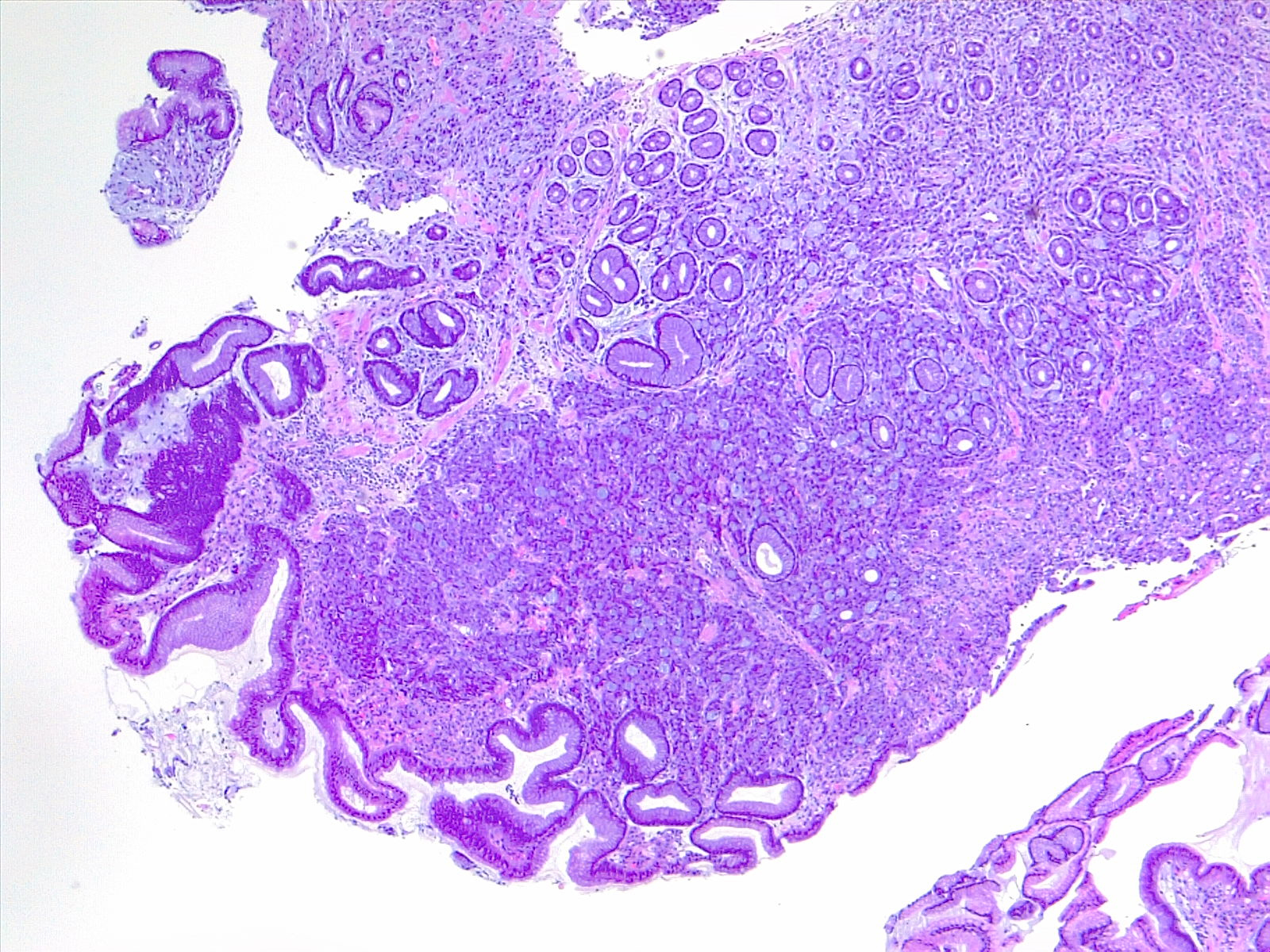
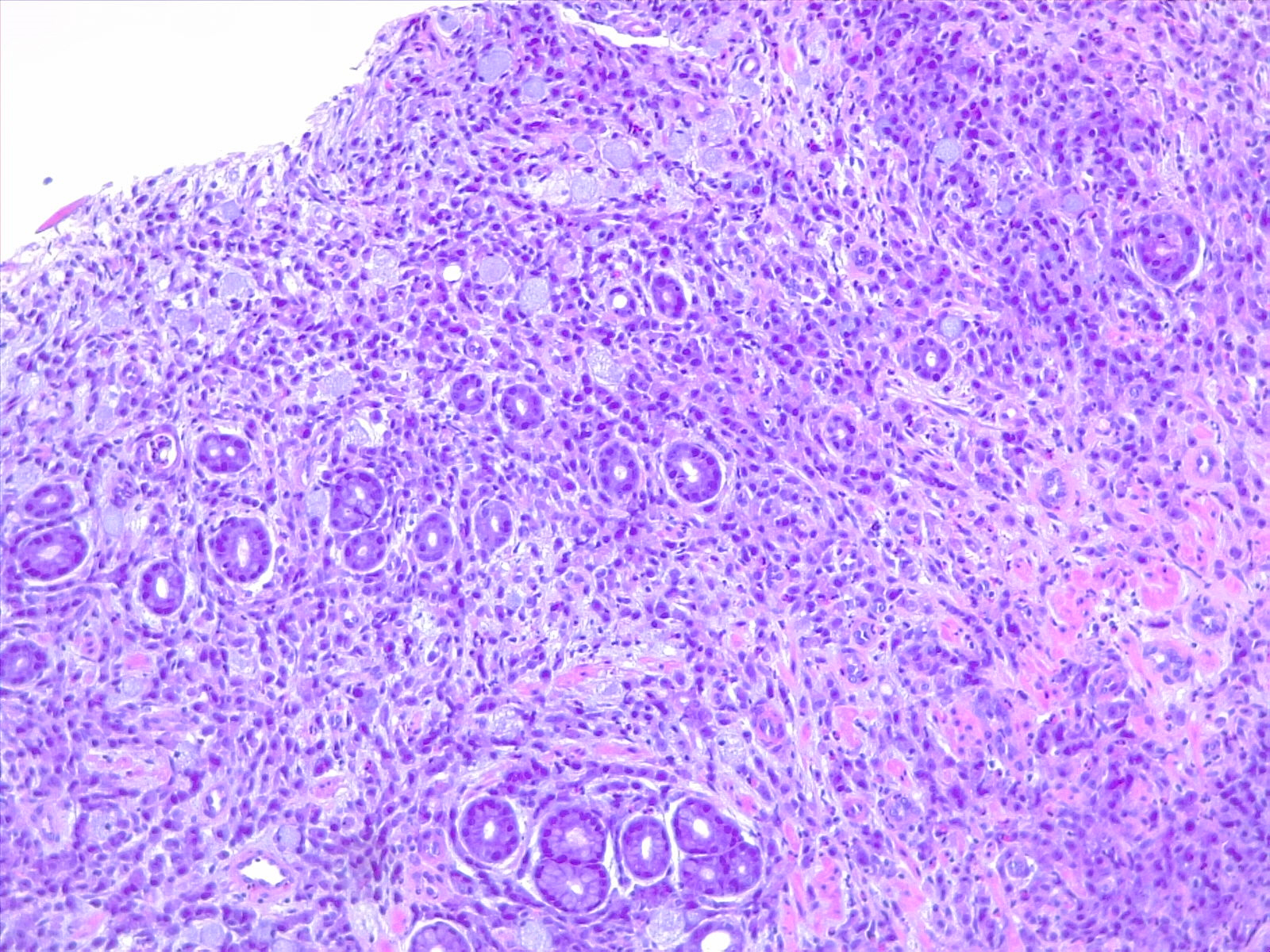
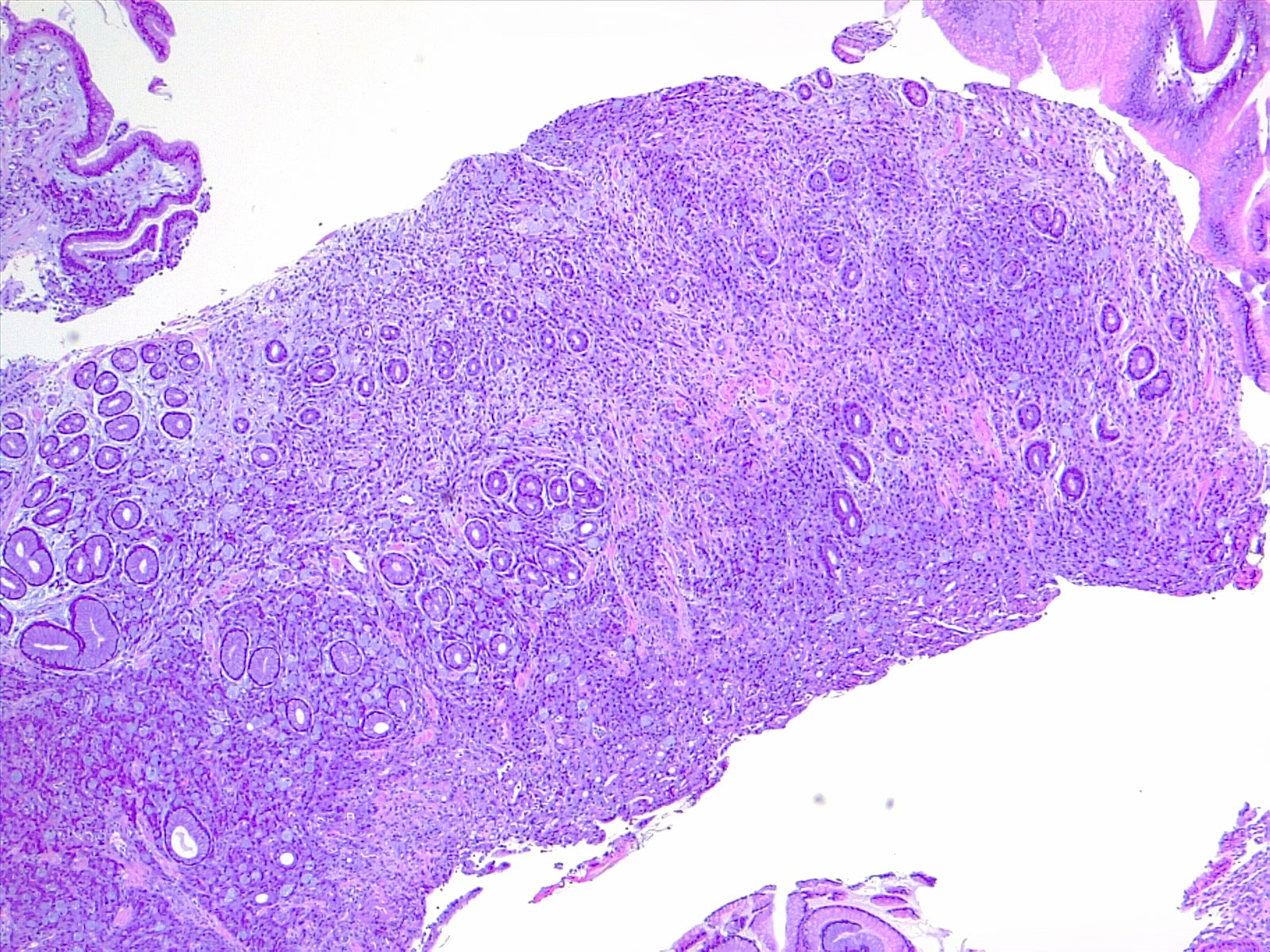
Etiology
Factors linked with an increased risk of gastric cancer include nutritional factors such as high-salt (salt-preserved food), N-nitroso compounds consumption (dietary source), smoking, a low vitamin A and C diet, consuming large amounts of smoked or cured foods, a deficit of refrigerated foods, and contaminated drinking water. High body mass index (BMI), increased calorie consumption, gastroesophageal reflux, and smoking are associated with an increased risk of adenocarcinomas of the distal esophagus, proximal stomach, and junction. Occupational exposure to rubber manufacturing, tin mining, metal processing, and coal also increases the risk. Helicobacter pylori infection has an attributable risk of 46% to 63%, while Epstein-Barr virus infection is an estimated 5% to 10% worldwide. Radiation exposure and prior gastric surgery also have been implicated as risk factors.
Diverse meta-analysis has shown that high consumption of fiber (relative risk (RR) 0.58 95% CI 0.49-0.67), fruits (RR 0.90, 95% CI 0.83-0.98 and vegetables (RR 0.96, 95% CI 0.88-1.06) have a probable protective benefit against gastric cancer. Aspirin and other non-steroidal anti-inflammatory agents have been associated with a lower risk of cancer of the gastroesophageal junction and other gastrointestinal tumors (HR 0.79 for each year of NSAID use). Alcohol consumption has not been demonstrated as a risk factor, and in fact, some data suggest daily wine intake may be protective despite insufficient evidence. Chronic “iatrogenic” histamine-2-receptor antagonist or proton pump inhibitor has not been associated with gastric cancer.
Host factors include type A blood with an approximate 20% more gastric cancer cases than in blood groups O, B, or AB and are particularly associated with the diffuse type. Pernicious anemia, an autoimmune chronic atrophic gastritis, has up to six-fold increased risk of intestinal-type gastric cancer. Benign gastric ulcers, hypertrophic gastropathy, and gastric polyps are risk factors associated with an increased risk of stomach cancer.
Most gastric cancers are sporadic, but 5% to 10% of cases have a family history of gastric cancer. Hereditary diffuse gastric cancer (HDGC), gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS), and familial intestinal gastric cancer (FIGC) are three major syndromes accounting for up to 3% to 5% of hereditary familial gastric cancer. Other hereditary cancer syndromes are:
- Hereditary non-polyposis colon cancer (HNPCC 13% lifetime risk, predominantly intestinal type)
- Familial adenomatous syndrome (FAP, 10% risk)
- Peutz Jeghers syndrome (PJS, 29% risk)
- Juvenile polyposis syndrome (JPS, 21%)
- Li-Fraumeni syndrome
- Hereditary breast and ovarian cancer syndrome
- Phosphatase and tensin homolog (PTEN) or hamartoma tumor (Cowden's) syndrome.
However, all these are rare causes of gastric cancer. It is recommended to follow screening guidelines on hereditary syndromes associated with gastric cancer according to their risk. Certain polymorphisms have been associated with gastric cancer. Carriers of IL-1B-511*T/*T or IL-1B-511*T/*C that are up-regulated by H. pylori infection could cause a pro-inflammation and acid inhibition leading to malignancy. Intestinal-type gastric carcinogenesis may have overexpressed oncogenes (K-ras and c-met) or tumor suppressors (TP53, APC, TTF, and CDKN1B,p27); although, not consistently present.
For cancer research, the World Health Organization has classified H. pylori as a definite gastric carcinogen and likewise concluded a positive association between the consumption of processed meat and stomach cancer.[2][3][4]
Evaluation
Patients presenting with any symptoms suspicious for gastric cancer should undergo an upper endoscopy over barium study (except for limited plastic presenting as leather-flask appearance). Although upper endoscopy is more invasive and costly, it offers tissue diagnosis by direct biopsy of esophageal, gastric, or duodenal lesions. Any suspicious gastric ulcer should be biopsied multiple times for higher diagnostic accuracy (one (70%) versus seven (98%) sensitivity). Gastric cancer screening by upper endoscopy has successfully detected early stages with higher curable rates after resection only in areas of high cancer incidence (Japan).
The American Joint Committee on Cancer/ Union for International Cancer Control (AJCC/UICC) Eight Edition 2017 has outlined a new staging scheme based on tumor, node, metastasis (TNM) with 5-year overall survival (5-y OS) according to pathological stage and intervention (surgery only IA-93.6%, IIA-81.8%, and IIIA-54.2% or with neoadjuvant I-76.5%, II-46.3%, III-18.3%, and IV-5.7%).
Staging pre-preoperative evaluations include chest and abdominal imaging to rule out metastasis and to determine surgical resectability. Abdominopelvic computerized tomography is performed early to rule out gross metastatic disease but does not accurately assess T, N, and small peritoneal metastases with an overall accuracy of 42% to 82%. Endoscopic ultrasound has a better diagnostic accuracy of tumor depth (57% to 88%) and lymph node status (30% to 90%), and hence, helps with accurate staging but is operator dependent. Biopsies should confirm suspicious solitary or oligometastatic sites; similarly, paracentesis should be performed if malignant ascites is suspected. Chest computerized tomography (CT) is preferred over plain radiograph. If prior staging evaluation is negative for the metastatic disease, positron emission tomography combined with computerized tomography imaging may help determine the resectability of gastric cancers in select cases (T2N0). Serum markers (carcinoembryonic antigen, glycoprotein CA 125 antigen, carbohydrate antigen 19-9, and cancer antigen 72-4) have limited utility and may be elevated due to other causes. Staging laparoscopy with peritoneal cytology analysis is indicated before surgery in the absence of visible spread, particularly for clinical stages with higher than T1b, and it is recommended for patients receiving preoperative therapy. Positive peritoneal cytology in the absence of identifiable peritoneal spread is an independent predictor of high recurrence after curative resection, and hence, surgery is not recommended.
Gastric cancer has reported human epidermal growth factor receptor 2 (HER2) gene amplification in 12% to 27% of cases and protein overexpression in 9% to 23% of cases. The impact of HER2 positivity remains largely unclear, but it has been implicated in tumor invasion and lymph node metastasis and is associated with poorer survival. HER2 positivity is more frequently found in intestinal subtype (33%) than diffuse (8%) with lower rates in the United States (19% and 6%, respectively). HER2 testing is recommended for all metastatic gastric cancer, first using immunohistochemistry score; negative for 0 or 1+ and positive for 3+, with reflex, fluorescent, in situ hybridization for an equivocal 2+ score to confirm. The United States Food and Drug Administration (FDA) has granted approval of immunotherapy for patients with microsatellite instability in solid tumors, including gastric cancer, and can evaluate the potential of immunotherapy in patients with metastatic disease who progressed on standard therapy. Gastric tumors positive for Epstein-Barr virus (EBV) have a better prognosis; however, staining for EBV is not yet recommended in routine clinical care.[11][12][13][14][15]
Treatment / Management
Treatment modality for gastric cancer depends on accurate preoperative staging. Therapeutic approach can be endoscopic resection for superficial, limited mucosa disease (< T1b, N0), upfront surgical resection with lymphadenectomy (< T3, any N), neoadjuvant (> T2) / adjuvant (> T1N1 or > T3N0) chemotherapy, radiation therapy, or combined with resectable lesions or palliative systemic therapy for those with locally advanced unresectable or metastatic disease (T4, any N, or M1).
Endoscopic Resection for Early Local Disease
Endoscopic resection either by endoscopic mucosal resection or endoscopic submucosal resection is offered to select patients with early gastric cancer with negative lymph nodes who meet selection criteria at centers of expertise. Standard selection criteria have a high- probability of en bloc resection, intestinal-type adenocarcinoma confined to the mucosa/submucosal, and absent venous or lymphatic invasion and tumors with diameters less than 20 mm without ulceration or 10 mm nonpolypoid flat or depressed lesions. Expanded criteria are under active investigation. Ten percent of mucosal and 20% of submucosal lesions will have lymph node metastasis and should be investigated carefully. If prior criteria are not met or an incomplete resection is performed, patients are referred for gastrectomy with regional lymph node resection. Successful endoscopic resection may offer a 5-year overall survival of 84% to 96% depending on the tumor's depth compared to gastrectomy survival rates up to 98%, but no randomized trials have compared both. Synchronous or metachronous gastric cancers can be found within 5 years in up to 9.2% of patients. H. pylori have been associated with metachronous gastric lesions, and eradication is recommended. Surveillance after endoscopic resection is the same strategy as for advanced cancer (detailed below).[16][17][18]
Surgical Resection for Resectable Disease
Patients with localized, resectable gastric cancer have the best chance of long-term survival with surgery alone. The main goal of surgery is complete resection with adequate margins (more than 4 cm), and only 50% of patients will obtain R0. Unresectability criteria are an invasion of major vasculature structure (aorta, hepatic artery, celiac axis, or proximal splenic artery), bulky adenopathy outside the surgical field, and the presence of linitis plastica; although, the latter is debatable. Most surgeons prefer total gastrectomy, but the technique depends on location, with proximal lesions requiring total resection and some distal lesions partial resection. Large mid-gastric lesions or diffuse disease should be offered a total gastrectomy. Routine or prophylactic splenectomy should be avoided. Standard surgical techniques in Japan, characterized by a better cancer survival, includes D2 resection (meticulous resection of all regional lymph nodes), which differs from the conservative type of lymphadenectomy performed in the United States, which carries less operative morbidity and mortality (standard D1 resection, removal of only perigastric lymph nodes). Two large trials by the Dutch Cancer Group and the Medical Research Council, comparing D1 with D2 lymphadenectomy, were flawed and underpowered to show D2 benefit. However, after a median 15-year follow-up of a 1078-patients in the randomized Dutch trial, D2 lymphadenectomy was associated with lower locoregional recurrence (12% versus 22%), regional recurrence (13% versus 19%), and gastric cancer–related death rates (37% versus 48%) than D1 surgery. Although D2 dissection was associated with significantly higher operative morbidity (10% versus 4%), complication rate (43% versus 25%), and higher reoperation rate (18% versus 8%) than D1 surgery. Considering a safer spleen-preserving D2 resection technique is currently available in high-volume centers, D2 lymphadenectomy is the recommended surgical approach for patients with resectable (curable) gastric cancer. D3 super-extended lymphadenectomy, including periaortic dissection, showed no added survival benefit with significantly worse perioperative complication rate in the multicenter Japan Clinical Oncology Group (JCOG) study 9501. While the optimal extent of lymphadenectomy is debated, current guidelines recommend 15 lymph nodes or more sampling, which showed a survival benefit. A high-volume center of excellence may offer laparoscopic resection instead of open gastrectomy, with a 5-year overall survival (OS) of 58.9% and 55.7%, respectively. Palliative resection, even with positive margins, is acceptable for symptomatic disease (obstruction or uncontrolled bleeding).[19][20]
Neoadjuvant and Adjuvant Therapy for Locally Advanced Resectable Disease
Surgical resection alone is potentially curative but only in early gastric cancer stages as seen in long-term survival rates on reported 5-year overall survival. It significantly declines from 75% for stage I to 35% for stage II and 25% or less for stage III, pushing research efforts to improve results using neoadjuvant (preoperative) or adjuvant (postoperative) therapies.
Neoadjuvant chemotherapy has been shown to downstage primary tumors and regional lymph nodes to attempt higher long-term curative resections. Neoadjuvant therapy should be offered to patients at high risk of developing distant metastases (bulky T3/T4, perigastric nodes, linitis plastica, or positive peritoneal cytology), sparing unnecessary surgery in case an emerging metastasis appears. The Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial was the first well-powered randomized clinical trial with 503 patients with resectable stomach cancer (74%), GEJ (15%), and distal esophagus (11%). Inclusion criteria were performance status 0-1, histological adenocarcinoma, and T2 or higher without distant metastasis. The MAGIC trial compared perioperative chemotherapy (consisted of three preoperative and three postoperative cycles of intravenous epirubicin [E: 50 mg per square meter of body-surface area] and cisplatin [C: 60 mg per square meter] on day 1, and a continuous intravenous infusion of fluorouracil [F: 200 mg per square meter per day] for 21 days) with surgery alone. Perioperative ECF chemotherapy significantly improved median overall survival (mOS HR, 0.75; 95% CI; 0.60, 0.93 [p = 0.009], 5-year overall survival rate, 36% versus 23%) and progression-free survival (PFS HR, 0.66; 95% CI; 0.53, 0.81 [p = < 0.001]) for patients. Worth noting, only about 55% of patients in the perioperative chemotherapy group received post-resection therapy. This suggests that the main therapy component responsible for the improved outcome was the preoperative treatment phase. Similar benefits for perioperative chemotherapy for CF was reported in the French FNLCC/FFCD trial that compared two to three cycles of preoperative and three to four postoperative chemotherapy (infusional FU 800 mg/m2 daily for 5 days plus cisplatin 100 mg/m2 on day 1 or 2, every 4 weeks) or surgery alone, resulting in a significantly better R0 (84% versus 73%), 35% reduction in disease-free survival (DSF) and 31% lower risk of death (5-year overall survival 38% versus 21%). EORTC trial 40954 failed to show a survival benefit of CF in the perioperative setting due to underpowered-limited accrual. Most recently, the FLOT4-AIO phase II/III trial compared the FLOT regimen (docetaxel [D: 50 mg/m2] plus oxaliplatin [O: 85 mg/m2] and leucovorin [L: 200 mg/m2] with short-term infusional fluorouracil [F: 2600 mg/m2 as a 24-hour infusion], all on day 1 and administered every 2 weeks) to an epirubicin-containing regimen (such as ECF [Magic trial protocol] or ECX [capecitabine X: 1250 mg/m by mouth, daily days 1 to 21 equivalent substitution REAL-2 trial]). In the phase, II FLOT regimen proved to have a higher pathologic complete response rate and less toxicity profile except for neutropenia. The phase III component enrolled 716 patients with resectable gastric cancer (44%) and GEJ (56%) with a preliminary report favoring FLOT mOS 50 months versus ECF/ECX 35 months (2017 annual meeting of the American Society of Clinical Oncology [ASCO]). There is no strong evidence regarding neoadjuvant chemoradiation over chemotherapy, becoming the focus of future research. The CALGB 80101, TROG 03.02, and TOPGEAR studies will evaluate whether chemoradiation therapy, in addition to perioperative chemotherapy, will be superior to the MAGIC approach alone.
Patients who have received potential curative resection without neoadjuvant therapy but are at high risk of recurrence should be offered adjuvant chemoradiation rather than surgery alone. The SWOG 9008/INT0116, a randomized phase III trial, assigned 556 patients with stages I to III gastroesophageal (20%) or gastric cancers (80%) to receive either surgery alone or surgery followed by adjuvant combined chemoradiotherapy. The combined therapy included the Macdonald regimen (one cycle of F; 425 mg/m2 per day and L; 20 mg/m2 per day) daily for 5 days followed 1 month later by 45 Gy (1.8 Gy/day) of radiation therapy (XRT), given with F; 400 mg/m2 per day and L; 20 mg/m2 per day on days 1 through 4 and the last 3 days of XRT, followed by two more 5-day cycles of F; 425 mg/m2 per day and L; 20 mg/m2 per day given at monthly intervals beginning 1 month after XRT completion). There was an approximate 20% improvement in survival for the group receiving the combined-modality therapy. The mOS in the surgery-only group was 27 months compared with 36 months in the chemoradiation group; the HR for death in the surgery-only arm was 1.35 (95% CI; 1.09, 1.66; p = 0.005) and relapse HR was 1.52 (95% CI; 1.23, 1.86; p < 0.001). The study has been criticized for the very low rate of D1 (< 54%) or D2 (< 10%) lymph node dissection, which was mandated per protocol. It is noticeable that the rate of distant metastasis was not reduced in the adjuvant chemoradiation group (16% versus 18%), suggesting that adjuvant therapy could have mainly compensated for inferior surgery. Despite the aforementioned, INT01116 established continued to demonstrate a persistent benefit after 10 years and became a new standard of care for patients with this disease in the United States in the adjuvant setting. CALGB Intergroup C80101 tried to improve the results obtained with INT0116 protocol regimen therapy by randomly selecting patients with resected gastric or GEJ cancer to receive chemoradiotherapy with ECF. This study was not adequately powered to assess non-inferiority and did not demonstrate any difference in outcomes between the two arms. The Korean Adjuvant Chemoradiotherapy in Stomach Tumors (ARTIST) trial provided further combined therapy evidence. This trial evaluated the addition of radiation therapy to adjuvant chemotherapy in patients who underwent gastrectomy with D2 lymph node dissection. Patients were randomly selected to receive six cycles of chemotherapy with CX (X; 2,000 mg/m2 per day on days 1 to 14 and C; 60 mg/m2 on day 1, repeated every 3 weeks) or two cycles of CX followed by chemoradiation (45 Gy XRT with concurrent daily X; 825 mg/m2 twice daily), followed by two more cycles of CX. At a median follow-up of 84 months, neither DFS nor OS was different between the two arms, although unplanned subsets of patients with node-positive disease and intestinal-type gastric cancer did have a significantly improved DFS with the addition of radiation therapy. The ongoing ARTIST2 trial will try to determine whether the addition of radiation therapy to adjuvant chemotherapy improves DFS in these subsets of patients at higher risk for recurrent disease.
In the United States, adjuvant chemotherapy is not frequently accepted and has not replaced combined therapy. Adjuvant chemotherapy alone was evaluated in the Japanese ACTS-GC phase III trial with S1 (an oral fluoropyrimidine 80 to 120 mg daily for 4 weeks, repeated every 6 weeks for 1 year) as postoperative D2-resection adjuvant therapy compared to surgery alone in 1059 patients with stage II or III gastric cancer and demonstrated a survival benefit with a 5-year OS rate of 72% in the S-1 group and 61% in the surgery only group (HR, 0.669; 95% CI, 0.540 to 0.828) and RFS (HR, 0.653; 95% CI, 0.537 to 0.793). By indirect comparison, the ACT-GC trial has better 5-year survival rates than INT0116 and MAGIC trials. The Korean phase III multicenter CLASSIC trial enrolled 1,035 patients with D2-resected stage II/III gastric cancer randomly assigned to adjuvant therapy with OX (eight 21-day cycles of X; 1000 mg/m2 twice daily on days 1 to 14 plus O; 130 mg/m2 on day 1) or surgery alone. Adjuvant chemotherapy had significant improvement in 3-year DFS (74% versus 59%; HR, 0.56 95% CI 0.44-0.72) and 5-year OS (78% versus 69%; HR, 0.66; 95% CI; 0.51, 0.85). In addition to these individual trials, an analysis of 34 trials with 724 patients confirmed that adjuvant chemotherapy without radiation after gastric cancer resection was associated with a significant OS benefit with an HR of 0.85 (95% CI; 0.80, 0.90) and an improvement in DFS (HR 0.79; 95% CI 0.72 to 0.87) versus surgery alone.
Standard of care varies according to regional preferences with postoperative therapy; chemoradiotherapy in the United States based on INT0116 trial, pre- and postoperative chemotherapy in the United Kingdom-based on MAGIC trial, or adjuvant chemotherapy alone after D2-resection in Korea and Japan based on ACT-GC and CLASSIC trial. Recently presented at ASCO 2016, the DUTCH CRITICS trial attempted to provide clarification by assigning 788 patients with potentially resectable stage Ib-IVa gastric cancers to receive three cycles of ECX or EOX followed by surgery later randomly allocated to three postoperative cycles of chemotherapy or chemoradiotherapy (45 Gy in 25 fractions with weekly cisplatin and daily capecitabine). Preliminary results showed no significant 5-year OS difference between chemotherapy and chemoradiotherapy (40.8 versus 40.9%). Of note, all patients had D1 or better resection, only 50% of patients completed full postoperative treatment, and toxicity profile was preferable in the chemotherapy alone group. Future enrollment in clinical trials is encouraged.[21][22][23][24][25][26][27][28][29]
Palliative Therapy for Locally Advanced Unresectable and Advanced Metastatic Disease
Unresectable locally advanced gastric cancer is often treated with advanced metastatic disease therapy regimens. The goals of medical treatment of advanced gastric cancer are primarily palliative symptoms, improve quality of life, and modest life-prolonging effect of weeks to months. Multiple agents are active in gastric cancer, including fluoropyrimidines (fluorouracil, capecitabine, and S1), anthracyclines (epirubicin), platinum agents (cisplatin and oxaliplatin), taxanes (paclitaxel and docetaxel), irinotecan, and other targeted therapies, including trastuzumab for HER2-overexpressing gastric cancers and ramucirumab, a VEGFR2 antibody. Combination regimens are associated with an increased response rate of up to 65% compared with single-agent therapies up to 40%.
All patients with advanced gastric cancer who are chemotherapy candidates should have their tumor evaluated for HER2 overexpression. HER2-positive patients can have trastuzumab added to a cytotoxic chemotherapy doublet backbone (most commonly fluoropyrimidine plus platinum). HER2-negative healthy patients can be offered doublet therapy (e.g., oxaliplatin plus leucovorin and infusional FU [FOLFOX], oxaliplatin plus capecitabine [XELOX], irinotecan plus leucovorin and FU [FOLFIRI] or FU plus cisplatin [CF]) over a triplet regimen (e.g., epirubicin plus cisplatin and infusional FU [ECF], epirubicin, cisplatin and capecitabine [ECX], epirubicin plus cisplatin and capecitabine [EOX], docetaxel, cisplatin, infusional FU [DCF] or modified DCF) due to treatment toxicities reserved only for highly selected patients with excellent performance status. Poor performance or elderly patients may be appropriate for single agents (e.g., leucovorin-modulated fluorouracil alone, single-agent capecitabine, single-agent irinotecan, or low-dose weekly taxanes). Docetaxel and irinotecan have shown a survival benefit for second-line chemotherapy. Nevertheless, there is no optimal standard of care.
Various fluorouracil-backbone combination trials established the additive benefit of anthracyclines (epirubicin), platinum agents (cisplatin and oxaliplatin), or taxanes (docetaxel) over older agents (mitomycin [FAM or MCF] or methotrexate [FAMTX]). A phase III German study trial showed similar survival benefit between the FLP regimen (fluorouracil, leucovorin, and cisplatin) and the FLO regimen (fluorouracil, leucovorin, and oxaliplatin); the later regimen had a significantly superior survival benefit in patients older than 65 compared to FLP. TAX-325 was a phase III clinical trial involving 445 chemotherapy-naïve patients with gastric cancer demonstrated the superiority of the addition of docetaxel to cisplatin and fluorouracil (DCF; 21-day cycles of C; 75 mg/m2 on day 1 plus F; 750 mg/m2 daily, days 1 to 5 and D; 75 mg/m2 on day 1) compared with cisplatin and fluorouracil alone (CF; 28-day cycles of C; 100 mg/m on day 1 plus F; 1000 mg/m per day days 1 to 5). DCF has a higher response rate (ORR, 37% versus 25%, p = 0.01), time to tumor progression (TTP, 5.6 months versus 3.7 months, p < 0.001), and mOS (9.2 months versus 8.6 months, p = 0.02). However, the addition of docetaxel is associated with significant toxicities, most notably, a high rate of febrile neutropenia (30% versus 14%) requiring supportive granulocyte-colony stimulating factor; therefore, this regimen is not advisable for patients with gastric cancer who have a poor performance status. The REAL-2 trial was a landmark large randomized phase III trial including 1,002 patients that compared four different chemotherapy regimens by substituting oral capecitabine (X) for continuous-infusion fluorouracil (F) and by using the non-nephrotoxic compound oxaliplatin (O) instead of cisplatin (C). The mOS in the ECF (control arm), ECX, EOF, and EOX groups were 9.9 months, 9.9 months, 9.3 months, and 11.2 months, respectively; survival rates at 1 year were 37.7%, 40.8%, 40.4%, and 46.8%, respectively. The combination of EOX was found to be less toxic and with significantly modestly longer mOS benefit compared to ECF (HR, 0.80; 95% CI; 0.66, 0.97 [p = 0.02]). Despite the previous trial results, the added value of an anthracycline (E) or taxane (D) component in the triplet treatment of advanced gastric cancer has been questioned; thus, a fluoropyrimidine /platinum chemotherapy doublet is still considered the standard of care by most experts. S1, when available, in combination with cisplatin, has also shown to be safe and effective (SPIRITS trial and FLAGS trial), but it remains investigational in The United States.
Irinotecan-based regimens (with fluoropyrimidine, cisplatin, or docetaxel) for advanced gastric cancer has shown no difference in outcome measures (ORR, PFS, and OS) when compared to non-irinotecan regimens (mainly FC or ECF). On comparison meta-analysis, the irinotecan-based regimen (FOLFIRI) was found to be less toxic, and there was a non-significant trend toward better survival with irinotecan (HR for death 0.86, 95% CI 0.73-1.02); thus, FOLFIRI could be offered as an alternative for those patients who are not considered candidates or unable to tolerate a platinum-based treatment regimen.
The role of different targeted agents added to cytotoxic backbone therapy has been investigated in several clinical trials. The first targeted agent with documented efficacy in advanced gastric and GEJ cancer was trastuzumab, the humanized monoclonal antibody against HER2. The phase III ToGA (trastuzumab in gastric cancer) trial compared six courses of cisplatin (80 mg/m2 on day 1) plus either infusional fluorouracil (F; 800 mg/m2/day on days 1 to 5) or capecitabine (X; 1,000 mg/m2 twice daily on days 1 to 14) based on physician choice with and without trastuzumab. Patients received trastuzumab 8 mg/kg loading dose, then 6 mg/kg every three weeks until disease progression on HER2-positive either by IHC 3+ or FISH + on a majority gastric cancer (80%) and GEJ (18%). Of the 3,807 tumors from patients with gastric cancer tested, 810 (22.1%) were positive for HER2 overexpression using immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) analysis. The addition of trastuzumab to FX increased mOS from 11.1 months to 13.8 months (HR, 0.74; 95% CI; 0.60, 0.91; p = 0.0046). In addition, secondary endpoints such as PFS (6.7 months vs. 5.5 months, p = 0.0002) and ORR (47.3% vs. 34.5%, p = 0.0017) were also improved in the trastuzumab arm. There were no significant differences in toxicity between the two treatment arms. The asymptomatic decrease in ejection fraction occurred in 4.6% in the trastuzumab arms, requiring baseline echocardiography and clinical monitoring. As a result of the ToGA trial, trastuzumab added to standard chemotherapy is the standard of care in patients with metastatic, HER2-overexpressing gastric, and gastroesophageal cancers. A higher trastuzumab dose (10mg/kg) did not improve efficacy in a phase III HELOISE trial. Contrary to the results obtains with trastuzumab, lapatinib (an oral HER2/EGFR kinase inhibitor) added to chemotherapy in HER2-overexpressing gastroesophageal cancers failed to meet survival benefit in the first-line (TRIO-01/LOGIC ) and second-line (TyTAN) settings. However, two pre-specified subgroups of the LOGIC trial patients, Asians, and those younger than 60 years, exhibited significant survival benefit with the addition of lapatinib to OX versus placebo/OX in the first-line therapy. Likewise, in the second-line setting evaluated in the TyTAN trial, patients with IHC3+ had significantly lower DFS and risk of death than those who received lapatinib plus paclitaxel. It is unclear if lapatinib can play a role in gastric cancer, given that trastuzumab has become the standard of care for HER2-positive gastroesophageal cancers.
Antibodies targeting the EGFR, a member of the HER family of receptor tyrosine kinases, such as cetuximab (EXPAND trial) and panitumumab (REAL-3 trial), have also been added to a chemotherapy backbone (CX and EOC, respectively) in gastroesophageal adenocarcinoma with worse toxicity profile and a detrimental effect on overall survival. The consistently negative data from two large phase III trials confirmed that cetuximab and panitumumab cannot be recommended in the first-line therapy advanced gastroesophageal adenocarcinoma.
Angiogenesis inhibitors in gastric and esophageal cancers have demonstrated inconsistent results. Promising data was obtained from a phase II study of bevacizumab added to cisplatin plus irinotecan with an improved response rate and median survival when compared to historical controls. However, in the first phase III trial (AVAGAST), bevacizumab failed to demonstrate an overall survival benefit (12.1 versus 10.1 months; HR, 0.87; 95% CI; 0.73, 1.03; p = 0.10), although, significantly improved PFS (6.7 versus 5.3 months; HR, 0.80; 95% CI; 0.68, 0.93; p = 0.0037) and ORR (46.0% versus 37.4%; p = 0.0315) when added to CF in patients with gastric adenocarcinomas. Due to noticeable geographical difference benefits in the Asian population, AVATAR study failed to improve mOS and PFS with the AVAGAST protocol in selected Chinese patients.
Ramucirumab, a different anti-angiogenesis VEGFR2 monoclonal antibody, was investigated in the second-line setting of advanced gastroesophageal cancer. Ramucirumab dosed at 8 mg/kg intravenously (IV) every 2 weeks showed a modest survival benefit of 9.6 months when combined with paclitaxel versus paclitaxel dosed at 80 mg/m on days 1, 8, and 15 of each 28-day cycle alone of 7.4 months at the RAINBOW trial (HR, 0.807; 95% CI; 0.678, 0.962; p = 0.017), and when used as a single agent (5.2 months) versus best supportive care (3.8 months) at the REGARD trial (HR, 0.776; 95% CI; 0.603, 0.998; p = 0.047), both in phase III clinical trial for patients with advanced gastroesophageal cancers who progressed first-line chemotherapy with a fluoropyrimidine and platinum agent. Of note, the REGARD trial had fewer Asian patients and higher GEJ in comparison to AVAGAST. Despite the benefit of ramucirumab seen in second-line trials, a randomized phase II study did not show a benefit to adding ramucirumab to mFOLFOX6 in the first-line setting for advanced gastroesophageal tumors.
After the failure of first-line fluoropyrimidine/platinum, the choice of second-line chemotherapy in the palliative management of gastric cancer can be either irinotecan or paclitaxel therapy with three randomized trials of chemotherapy versus best supportive care clearly demonstrated improvement in overall survival or neither is superior. Radiation therapy alone can be effective for symptomatic control, but it is rarely used alone to treat primary advanced unresectable gastric cancer.
Several novel agents are currently being investigated in advanced gastric cancer. A phase I clinical trial selected patients with low expression of ataxia-telangiectasia protein had a significant survival benefit from paclitaxel plus olaparib (an oral active poly-ADP ribose polymerase inhibitor) with mOS not reached when compared to paclitaxel plus placebo of 8.2 months, pending an undergoing phase III trial to confirm results. Other promising agents are immune checkpoint inhibitors, particularly anti-PD-1 monoclonal antibody pembrolizumab. KEYNOTE-012 trial evaluated pembrolizumab in patients PD-L1 positive tumors on heavily treated metastatic gastric cancer (and other advanced carcinomas) showing median response duration of 40 weeks and mOS 11.4 months, higher when compared to historical 5.2 months REGARD study. Advanced Gastric and GEJ PD-L1 expressing carcinoma were explored by the KEYNOTE-059 cohort showing an ORR of 13% (95% CI: 8.2,20.0) with a 58% having a response duration of 6 months or longer, obtaining an FDA accelerated approval. The frequency of microsatellite instability-high (MSI-H) and deficient mismatch repair (dMMR), which are susceptible to immunotherapy, has been reported as high as 16% in gastric adenocarcinomas. Eighty-six selected dMMR patients with 12 different tumor types (including refractory advanced or metastatic gastroesophageal carcinoma) who received pembrolizumab achieved a 53% objective response rate and a 21% complete response, further resulting in a subsequent FDA approval for any advanced solid tumor with these genetic features and non-satisfactory alternative. Other second-line targeted therapy agents with clinically improved survival outcomes in clinical trials are apatinib (an oral VEGFR-2 inhibitor with median 6.5 versus 4.7 months placebo) and regorafenib (an oral multikinase inhibitor with median 5.8 versus 4.5 months placebo), demonstrating promise as salvage therapy for this patient population. [30][31][32][33][34][35][36][37][38][39][40]